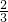��Ŀ����
�̽���������Ҫ��ũ��Ʒ֮һ����һЩ�ط����ڻ���ʩ�ò��������Ը̽�Ʒ�ʡ������ṹ����ɲ���Ӱ�죬���Ҫ�ᳫ��ѧʩ�ʣ�����ũ�ҷ��ϣ����ľ�ң�����Ч�ɷ���K2CO3���ȣ�ij��ȤС��Ϊ�˲ⶨ��ľ����K2CO3�ĺ���������K2CO3��������ϡ���ᷴӦ����ʵ�飬��ӦʽΪ��K2CO3+2HCL�T2KCI+H2O+C02���й��������±���
| ��ȡ��ľ����Ʒ���������ˣ� | ϡ������HCl���������� | �ռ���CO2�������ˣ� |
| 50 | 10% | 2 |
��1����Ӧʱʵ������ϡ����������Ƕ��ٿˣ�
��2���ò�ľ���к�K2CO3�����������Ƕ��٣�
��3��ʩ�ò�ľ�ң���Ҫ��ʹ���������Ӽ�Ԫ�أ�����ij��ʩ��������ľ��50ǧ�ˣ����൱�����������Ӷ���ǧ�˵ļ�Ԫ�أ�
�⣺�跴Ӧ�����ĵ�HCl������Ϊx����ľ����K2CO3������Ϊy
K2CO3+2HCl=2KCl+H2O+CO2��
138 73 44
y x 2g
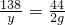
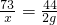
y=6.27g x=3.32g
����ϡ����������� =33.2g
=33.2g
��ľ���к�K2CO3����������= ��100%=12.54%
��100%=12.54%
ʹ�����������˼�Ԫ�ص�����Ϊ��50ǧ�ˡ�12.54%�� ��100%=3.54ǧ��
��100%=3.54ǧ��
�𣺣�1������ϡ�����������33.2��
��2����ľ���к�K2CO3����������Ϊ12.54%
��3��ʹ�����������˼�Ԫ��3.54ǧ��
��������1������̼��������ᷴӦ�Ļ�ѧ����ʽ�����ݶ�����̼����������μӷ�Ӧ��ϡ���������ʵ��������������ϡ�����������
��2������̼��������ᷴӦ�Ļ�ѧ����ʽ�����ݶ�����̼���������̼��ص�������
��3����ľ�ҵ���������ľ����̼��ص���������=̼��ص�������̼��ص�������̼����м�Ԫ�ص���������=��Ԫ�ص�������
������������Ҫ������ѧ���û�ѧ֪ʶ���ʵ����������������鷽ʽ�Ƚ�������ؼ���Ҫ���պ��й����������ļ��㣮
K2CO3+2HCl=2KCl+H2O+CO2��
138 73 44
y x 2g
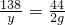
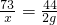
y=6.27g x=3.32g
����ϡ�����������
 =33.2g
=33.2g��ľ���к�K2CO3����������=
 ��100%=12.54%
��100%=12.54%ʹ�����������˼�Ԫ�ص�����Ϊ��50ǧ�ˡ�12.54%��
 ��100%=3.54ǧ��
��100%=3.54ǧ���𣺣�1������ϡ�����������33.2��
��2����ľ���к�K2CO3����������Ϊ12.54%
��3��ʹ�����������˼�Ԫ��3.54ǧ��
��������1������̼��������ᷴӦ�Ļ�ѧ����ʽ�����ݶ�����̼����������μӷ�Ӧ��ϡ���������ʵ��������������ϡ�����������
��2������̼��������ᷴӦ�Ļ�ѧ����ʽ�����ݶ�����̼���������̼��ص�������
��3����ľ�ҵ���������ľ����̼��ص���������=̼��ص�������̼��ص�������̼����м�Ԫ�ص���������=��Ԫ�ص�������
������������Ҫ������ѧ���û�ѧ֪ʶ���ʵ����������������鷽ʽ�Ƚ�������ؼ���Ҫ���պ��й����������ļ��㣮

��ϰ��ϵ�д�
 ���������ʱ��ѵϵ�д�
���������ʱ��ѵϵ�д� �㽭�¿γ���άĿ�������ʱ��ѵϵ�д�
�㽭�¿γ���άĿ�������ʱ��ѵϵ�д� ��������ϵ�д�
��������ϵ�д� ���ɶ���ܲ��¿�ֱͨ�߿�ϵ�д�
���ɶ���ܲ��¿�ֱͨ�߿�ϵ�д�
�����Ŀ
����ͼ��Ԫ�����ڱ���һ���֣����ֱ�ʾ��ӦԪ�ص�ԭ�������������в���Ԫ�ص�ԭ�ӣ����ӣ��ṹʾ��ͼ���£�
| 1H | |||||||
| 8O | |||||||
| 12Mg | 17Cl | ||||||
��2��ѡ�ñ���Ԫ����գ�A2B2�ͻ�����Ļ�ѧʽ��________��ij�ͻ��������Mg��O��ɵĻ����д���仯ѧʽ________��
M��һ�ֻ�Ա���ǿ�Ľ�����M���ӣ�M2+���������������ӹ��ɵĻ�������ܽ��Լ��±���
| O2- | OH - | CO32- | ��Clһ | SO42- | NO3- | |
| M 2+ | ���� | ���� | ���� | ���� | ���� | ���� |
��M��0H��2+NaCl����M��0H��2+HCl������M��NO3��2+HCl����MCO3+HCl
��MSO4+BaCl2
���в���ֱ�ӷ�Ӧ��ȡMCl2����
- A.�ڢܢ�
- B.�ݢޢߢ�
- C.�٢ڢܢ�
- D.�٢ۢݢ�