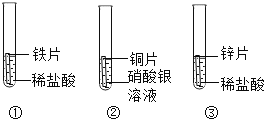
ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩœθΥαΦΊ”ꬻ̷ֺΒΡ»ήΫβΕ»«ζœΏ»γΆΦΥυ ΨΘ§«κΜΊ¥πœ¬Ν–Έ ΧβΓΘ
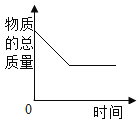
(1)t1Γφ ±Θ§ΝΫΈο÷ ÷–»ήΫβΕ»Ϋœ¥σΒΡ «________;
(2)t2Γφ ±Θ§ΝΫΈο÷ ΒΡΒ»÷ ΝΩ±ΞΚΆ»ή“ΚΫΒΈ¬÷Νt1ΓφΘ§Ιΐ¬ΥΘ§ΥυΒΟœθΥαΦΊ»ή“Κ÷ ΝΩ__________ΥυΒΟ¬»Μ·ΦΊ»ή“Κ÷ ΝΩ;(ΧνΓΑΘΨΓ±ΓΔΓΑΘΫΓ±ΜρΓΑΘΦΓ±)
(3)t2 Γφ ±Θ§ΫΪ100gΥ°Φ”»Υ Δ”–50g¬»Μ·ΦΊΒΡ…’±≠÷–Θ§≥δΖ÷»ήΫβΚσΘ§ΒΟΒΫ»ή“Κ÷ ΝΩΈΣ_______g;
(4)”ϊ≈δ÷Τ»ή÷ ΒΡ÷ ΝΩΖ÷ ΐΈΣ20%ΒΡœθΥαΦΊ»ή“ΚΘ§”Π¬ζΉψΒΡΈ¬Ε»ΖΕΈß «__________ΓΘ
ΓΨ¥πΑΗΓΩ ¬»Μ·ΦΊ < 140g Έ¬Ε»≤ΜΒΆ”Ύt1ΓψC
ΓΨΫβΈωΓΩΘ®1Θ©”…»ήΫβΕ»«ζœΏΩ…÷ΣΘ§t1Γφ ±Θ§ΝΫΈο÷ ÷–»ήΫβΕ»Ϋœ¥σΒΡ «¬»Μ·ΦΊΘΜ
Θ®2Θ©t2Γφ ±Θ§œθΥαΦΊΒΡ»ήΫβΕ»¥σ”Ύ¬»Μ·ΦΊΒΡ»ήΫβΕ»Θ§t1Γφ ±¬»Μ·ΦΊΒΡ»ήΫβΕ»¥σ”ΎœθΥαΦΊΒΡ»ήΫβΕ»Θ§Ι t2Γφ ±Θ§ΝΫΈο÷ ΒΡΒ»÷ ΝΩ±ΞΚΆ»ή“ΚΫΒΈ¬÷Νt1ΓφΘ§œθΥαΦΊ»ή“Κ÷–Έω≥ωΒΡΨßΧεΕύΘ§Ιΐ¬ΥΘ§ΥυΒΟœθΥαΦΊ»ή“Κ÷ ΝΩ–Γ”Ύ¬»Μ·ΦΊ»ή“ΚΒΡ÷ ΝΩΘΜ
Θ®3Θ©t2 Γφ ±Θ§¬»Μ·ΦΊΒΡ»ήΫβΕ»ΈΣ40gΘ§Κ§“ε «÷Η‘Ύt2 Γφ ±100gΒΡΥ°÷–ΉνΕύΩ…»ήΫ⬻̷ֺΒΡ÷ ΝΩΈΣ40gΘ§Ι Φ”»κ50gΒΡœθΥαΦΊΚσΘ§”–10gΒΡœθΥαΦΊ≤ΜΡή»ήΫβΘ§Ι ΥυΒΟ»ή“ΚΒΡ÷ ΝΩΈΣ140gΘ§
Θ®4Θ©…η‘Ύ100gΥ°÷–Φ”»κxgΒΡœθΥαΦΊΩ…≈δΒΟ20%ΒΡœθΥαΦΊ»ή“ΚΘ§ ![]() =20%Θ§ΒΟx=25gΘ§œθΥαΦΊΒΡ»ήΫβΕ»ΈΣ25g ±Ε‘”ΠΒΡΈ¬Ε»ΈΣt1ΓφΘ§Ι Έ¬Ε»≤ΜΒΆ”Ύt1ΓψCΘΜ
=20%Θ§ΒΟx=25gΘ§œθΥαΦΊΒΡ»ήΫβΕ»ΈΣ25g ±Ε‘”ΠΒΡΈ¬Ε»ΈΣt1ΓφΘ§Ι Έ¬Ε»≤ΜΒΆ”Ύt1ΓψCΘΜ

ΓΨΧβΡΩΓΩΆ§―ßΟ«Ω…“‘Ά®Ιΐœ¬Ν–ΖΫ Ϋ»œ ΕΩ’ΤχΓΘ
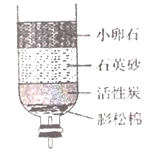
ΓΨΉι≥…Ϋ«Ε»ΓΩ
ΔΌΩ’Τχ÷–ΧεΜΐΖ÷ ΐ‘ΦΈΣ78%ΒΡΈο÷ «___________ΓΘ
ΔΎΈΣ≤βΕ®Ω’Τχ÷–―θΤχΧεΜΐΖ÷ ΐΘ§…ηΦΤœ¬ΆΦ Β―ιΓΘ
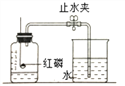
Δώ.ΈΣΝΥ»Ζ±Θ Β―ι≥…ΙΠΘ§‘ΎΉΑ“©ΤΖ÷°«Α”ΠΗΟΦλ≤ιΉΑ÷ΟΒΡ _________ΘΜ
Δρ.ΗΟ Β―ι÷–ΚλΝΉ–η“ΣΙΐΝΩΒΡ‘≠“ρ «__________ΘΜ
Δσ.ΚλΝΉ»Φ…’ΒΡœ÷œσ «____________Θ§Ζ¥”ΠΒΡΜ·―ßΖΫ≥Χ Ϋ ____________ΘΜ
Δτ.ά以÷Ν “Έ¬Κσ¥ρΩΣ÷ΙΥ°Φ–Ιέ≤λΒΫΒΡœ÷œσ « _______________ΘΜ”…¥ΥΒΟ≥ωΩ’Τχ÷–―θΤχΒΡΧεΜΐΖ÷ ΐ‘ΦΈΣ ___________ΓΘ
ΓΨΈΔΙέΫ«Ε» ΓΩ
ΔΌ”ΟΓΑΜ·―ßΖϊΚ≈Γ±ΜρΓΑΆΦ ΨΓ±ΧνΩ’ΓΘ
![]()
ΆΦ Ψ |
| ______ |
|
Μ·―ßΖϊΚ≈ | ______ | N2 | _______ |
ΔΎΆ§Έ¬Ά§―Ιœ¬Θ§ΤχΧεΒΡΧεΜΐ±»Β»”ΎΖ÷Ή”Ηω ΐ±»ΓΘ»τΚω¬‘Ω’Τχ÷–ΤδΥϋ≥…Ζ÷Θ§»γΆΦΩ…±μ ΨΩ’ΤχΈΔΙέΡΘ–ΆΒΡ «__________(Χν―Γœν)ΓΘ
ΓΨ±δΜ·Ϋ«Ε»ΓΩ
‘Ύ“ΜΗω±ξΉΦ¥σΤχ―Ιœ¬Θ§Ω’Τχ÷–≤ΩΖ÷ΉιΖ÷ΒΡΖ–Βψ»γœ¬ΘΚ
ΉιΖ÷ | ΒΣΤχ | ―θΤχ | Εΰ―θΜ·ΧΦ |
Ζ–Βψ(Γφ) | -195.8 | -183.0 | -78.4 |
ΔΌΫΪ»ΦΉ≈ΒΡΡΨΧθ÷Ο”Ύ Δ”–±μ÷–ΉιΖ÷ΒΡΜλΚœ“ΚΒΡΗ÷ΤΩΩΎΘ§Ιέ≤λΒΫΒΡœ÷œσ «____________ΓΘ
ΔΎœ¬Ν––π ω¥μΈσΒΡ «_____________ΓΘ
AΘ°ΡΨΧΩ‘Ύ―θΤχ÷–»Φ…’Θ§ΖΔ≥ωΑΉΙβ
BΘ°ΧζΥΩ‘ΎΩ’Τχ÷–»Φ…’Θ§Μπ–«ΥΡ…δΘ§…ζ≥…ΚΎ…ΪΙΧΧε
CΘ°¥”±υœδάοΡΟ≥ωΤϊΥ°Θ§ΤΩΉ”Άβ±μ”–“Κ÷ιΘ§ΥΒΟςΩ’Τχ÷–”–Υ°’τΤχ
DΘ°ΨΟ÷Ο≥Έ«ε ·Μ“Υ°ΒΡ ‘ΦΝΤΩΡΎ±Ύ”–“Μ≤ψΑΉΡΛΘ§÷ΛΟςΩ’Τχ÷–”–Εΰ―θΜ·ΧΦ
ΔέΧζΥΩ‘Ύ―θΤχ÷–»Φ…’ΒΡΜ·―ßΖΫ≥Χ Ϋ «____________ΓΘ
ΓΨ”Π”ΟΫ«Ε»ΓΩ
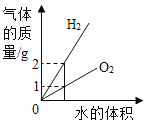
ΔΌΨΤΨΪ(C2H5OH) «“Μ÷÷≥Θ”ΟœϊΕΨΦΝΘ§ΨΤΨΪ”…___________÷÷‘ΣΥΊΉι≥…Θ§Τδ÷–«β‘ΣΥΊ”κ―θ‘ΣΥΊΒΡ÷ ΝΩ±»ΈΣ_______ΘΜ«β‘ΣΥΊΒΡ÷ ΝΩΖ÷ ΐΈΣ________(Ω…”ΟΖ÷ ΐ±μ Ψ)ΘΜΟΩΗωΨΤΨΪΖ÷Ή”Κ§________Ηω‘≠Ή”ΘΜ46gC2H5OH÷–Κ§_________Ηω―θ‘≠Ή”ΓΘ≈δΤΫΨΤΨΪ»Φ…’ΒΡΜ·―ßΖΫ≥Χ ΫΒΡœΒ ΐ“ά¥ΈΈΣ_______ΓΘ
ΓθC2H5OH+ΓθO2![]() ΓθCO2+ΓθH2O
ΓθCO2+ΓθH2O
ΔΎ ≥ΤΖΑϋΉΑΡΎ≥δN2“‘ΖάΗ·Θ§“ρΈΣN2ΒΡΜ·―ß–‘÷ _____________ΘΜ
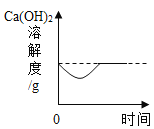
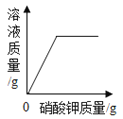
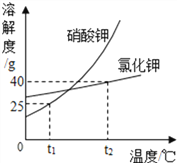
ΓΨΧβΡΩΓΩœ¬±μ ««β―θΜ·ΗΤΚΆ«β―θΜ·ΡΤΒΡ»ήΫβΕ» ΐΨίΓΘ«κΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Έ¬Ε»/Γφ | 0 | 20 | 40 | 60 | 80 | 100 | |
»ήΫβΕ» (g/100gH2O) | «β―θΜ·ΗΤ | 0.19 | 0.17 | 0.14 | 0.12 | 0.09 | 0.08 |
«β―θΜ·ΡΤ | 31 | 91 | 111 | 129 | 313 | 336 | |
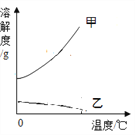
ΔΌ «β―θΜ·ΡΤ»ήΫβΕ»«ζœΏ «____Θ®―ΓΧνΓΑΦΉΓ±ΜρΓΑ““Γ±Θ©
ΔΎ Α―Ϋ”Ϋϋ±ΞΚΆΒΡ«β―θΜ·ΗΤ»ή“Κ±δ≥…±ΞΚΆ»ή“ΚΒΡΖΫΖ® «______ΓΘ
Δέ 20 Γφ ±Θ§10 gΥ°÷–»ήΫβ______ΩΥ«β―θΜ·ΡΤ«ΓΚΟ±ΞΚΆΓΘ
Δή 20 Γφ ±Θ§Ζ÷±π‘Ύ100ΩΥΥ°÷–Φ”»κmΩΥ«β―θΜ·ΡΤΚΆ«β―θΜ·ΗΤΙΧΧεΘ§Ω…ΒΟΒΫ÷ ΝΩΖ÷ ΐœύΆ§ΒΡΝΫ÷÷»ή“ΚΘ§‘ρmΒΡ»Γ÷ΒΖΕΈß «______ΓΘ
Δί 60 Γφ ±Θ§«β―θΜ·ΡΤΒΡ±ΞΚΆ»ή“Κ÷–Κ§…ΌΝΩ«β―θΜ·ΗΤΘ§Α―ΤδΫΒΈ¬Θ§ΫαΨßΚσΙΐ¬ΥΘ§Ε‘¬Υ‘ϋΓΔ¬Υ“Κ≥…Ζ÷Ζ÷Έω’ΐ»ΖΒΡ «______ΓΘ
Δώ.¬Υ‘ϋ÷–÷Μ”–«β―θΜ·ΡΤ
Δρ.¬Υ‘ϋ÷–“ΜΕ®”–«β―θΜ·ΡΤΘ§Ω…Ρή”–«β―θΜ·ΗΤ
Δσ.¬Υ“Κ÷–“ΜΕ®”–«β―θΜ·ΗΤΘ§Ω…Ρή”–«β―θΜ·ΡΤ
Δτ.¬Υ“Κ÷–“ΜΕ®”–«β―θΜ·ΡΤΚΆ«β―θΜ·ΗΤ
Δό «β―θΜ·ΗΤ»ή“ΚΚΆ«β―θΜ·ΡΤ»ή“ΚΕΦ «Έό…Ϊ»ή“ΚΘ§ΕΦΡή”κΕΰ―θΜ·ΧΦΖΔ…ζΖ¥”ΠΓΘ«β―θΜ·ΡΤ”κΕΰ―θΜ·ΧΦΖ¥”ΠΒΡΖΫ≥Χ ΫΈΣ: 2NaOH + CO2![]() Na2CO3+H2OΓΘ«β―θΜ·ΗΤ”κΕΰ―θΜ·ΧΦΖ¥”ΠΒΡΖΫ≥Χ ΫΈΣ______________ΓΘ
Na2CO3+H2OΓΘ«β―θΜ·ΗΤ”κΕΰ―θΜ·ΧΦΖ¥”ΠΒΡΖΫ≥Χ ΫΈΣ______________ΓΘ
ΗυΨί“‘…œ–≈œΔΘ§Ρψ»œΈΣ____Θ®―ΓΧνΓΑΡήΓ±ΜρΓΑ≤ΜΡήΓ±Θ©”ΟΕΰ―θΜ·ΧΦά¥Φχ±π’βΝΫ÷÷»ή“ΚΓΘ
ΓΨΧβΡΩΓΩ»γΆΦΥυ ΨΆΦœώ÷–Θ§Ρή’ΐ»ΖΖ¥”≥Ε‘”Π±δΜ·ΙΊœΒΒΡ «
A | B | C | D |
|
|
|
|
œρ±ΞΚΆ ·Μ“Υ°÷–Φ”»κ“ΜΕ®ΝΩCaO | “ΜΕ®Έ¬œ¬Θ§œρ≤Μ±ΞΚΆœθΥαΦΊ»ή“Κ÷–Φ”»κœθΥαΦΊΙΧΧε | “ΜΕ®÷ ΝΩΒΡΚλΝΉ‘ΎΟή±’»ίΤςΡΎ»Φ…’ | ΒγΫβΥ°…ζ≥…«βΤχΚΆ―θΤχ÷ ΝΩ |
A. A B. B C. C D. D