��Ŀ����
����������������Ӧ�ù㷺��
(1)�����ˮ�ܲ���������Ŀǰ�������Ϲܺ����Ͻ������Ϲܣ�������ˮһ����ͭ�ܡ����йܲ��У����ڽ������ϵ���______ ����ĸ���
����ĸ��� ��
��

(2)���������г��õĽ�������ͼ��ij��ȡůƬ�����װ��ͼƬ���á�ȡůƬ���з��ȼ���Ҫ�ɷ������ۡ�����̿���Ȼ��ơ�ˮ�ȣ��䷢��������������ʱ�ᷢ�ȡ�

 ���ȼ���Ӵ������Żᷢ�ȣ�ԭ������Ҫ��______�����ʹ�ͬ���òŻ����⡣
���ȼ���Ӵ������Żᷢ�ȣ�ԭ������Ҫ��______�����ʹ�ͬ���òŻ����⡣
 �Ʋⷢ�ȼ��ɷ����Ȼ��Ƶ�����______�����������Ƭ����������Ȼ�ͭ�Ļ����Һ�У����ܷ������û���Ӧ�Ļ�ѧ����ʽ��______��
�Ʋⷢ�ȼ��ɷ����Ȼ��Ƶ�����______�����������Ƭ����������Ȼ�ͭ�Ļ����Һ�У����ܷ������û���Ӧ�Ļ�ѧ����ʽ��______��
��ϰ��ϵ�д�
 �п������п��Ծ����ϵ�д�
�п������п��Ծ����ϵ�д� ��������״Ԫ��ϵ�д�
��������״Ԫ��ϵ�д�
�����Ŀ


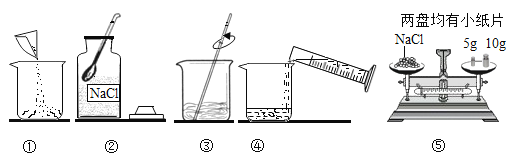
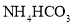 �����Ʊ�̼�����ƺ��Ȼ�泥�
�����Ʊ�̼�����ƺ��Ȼ�泥�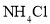 �����÷�Ӧ�ɱ�ʾΪ��
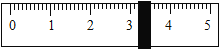
�����÷�Ӧ�ɱ�ʾΪ�� ��20��ʱ����������ѧ����ʽ�з�Ӧ��������ȣ���100gˮ�м���11.7gNaCl��15.8g
��20��ʱ����������ѧ����ʽ�з�Ӧ��������ȣ���100gˮ�м���11.7gNaCl��15.8g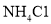
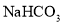

 B.
B.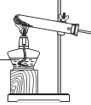 C.
C. D.
D.
 C+D ����������˵������������
C+D ����������˵������������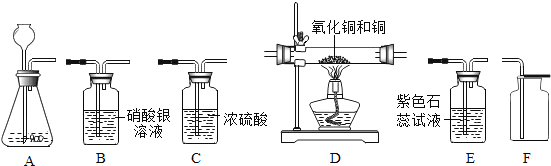
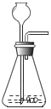
 Cu+H2O������C1��C2��C3ΪŨ����ϴ��ƿ����������װ�õ������ԣ�����Ƶ�ʵ��װ�������������Ǵ������ҡ�
Cu+H2O������C1��C2��C3ΪŨ����ϴ��ƿ����������װ�õ������ԣ�����Ƶ�ʵ��װ�������������Ǵ������ҡ�