��Ŀ����
CO2�������Ʊ�̼�����ϣ��������������У�ijͬѧ�������ʵ�飬�ⶨijƷ��̼�������е�CO2�ĺ�����
ʵ�鷽��һ��
�ٽ�250mLƿװ̼�����Ϸ��ڱ������䶳���պý����
�ڽ����ϴӱ�����ȡ����Ѹ�ټ�����������Ϊ50%NaOH��Һ5mL����ת����ƿ��������Ȼ����ûָ������£����ⶨ��
�۳Ƶ�װ��C������Ϊx g����ͼ1����ʵ��װ�ã�ȡ50mL����Һ���������У��رտ���a���������ٵĻ���������������ע��ϡ���ᣬ�����ٲ������ݣ��ر������ٵĻ�����
�ܴ���a����װ���й��������һ��ʱ�����Cװ������Ϊy g��
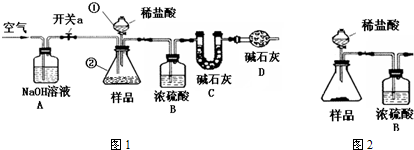
ע��ʵ���и�����Һ���ܶȶ���Ϊ1g/mL����ʯ����CaO��NaOH�Ĺ������
��1������װ���У������ٵ�����Ϊ
��2��װ��A��Ŀ����
��3��������䶳̼�����ϵ�Ŀ����
��4������ܹ��������Ŀ����
��5�����������ṩ�����ݼ����Ʒ��̼��������CO2�ĺ���Ϊ
��6����û��Bװ�ã�����CO2������
ʵ�鷽������
�٢��뷽��һ��ͬ���۰�ͼ2����ʵ��װ�ã��Ƶ�����װ�ã���ҩƷ��������������ע������ϡ���ᣬ���10s������������װ�õ�������ֱ������װ�õ���������Ϊֹ��
��1��ע������ϡ��������Ļ�ѧ��Ӧ����ʽΪ
��2����ʵ���г����������ٽ���
��3��װ��B��Ŀ����
��4����ͬѧ����÷������CO2������ƫС����ͬѧ�����ɳ��˿����ǿ�����ˮ������ʵ������Ӱ���⣬��������
ʵ�鷽��һ��
�ٽ�250mLƿװ̼�����Ϸ��ڱ������䶳���պý����
�ڽ����ϴӱ�����ȡ����Ѹ�ټ�����������Ϊ50%NaOH��Һ5mL����ת����ƿ��������Ȼ����ûָ������£����ⶨ��
�۳Ƶ�װ��C������Ϊx g����ͼ1����ʵ��װ�ã�ȡ50mL����Һ���������У��رտ���a���������ٵĻ���������������ע��ϡ���ᣬ�����ٲ������ݣ��ر������ٵĻ�����
�ܴ���a����װ���й��������һ��ʱ�����Cװ������Ϊy g��

ע��ʵ���и�����Һ���ܶȶ���Ϊ1g/mL����ʯ����CaO��NaOH�Ĺ������
��1������װ���У������ٵ�����Ϊ
��Һ©��
��Һ©��
�������ڵ�����Ϊ��ƿ
��ƿ
����2��װ��A��Ŀ����
���տ����еĶ�����̼��ˮ
���տ����еĶ�����̼��ˮ
��װ��D��Ŀ������ֹ�����еĶ�����̼��ˮ���룬Ӱ��ʵ����
��ֹ�����еĶ�����̼��ˮ���룬Ӱ��ʵ����
����3��������䶳̼�����ϵ�Ŀ����
��ֹ������̼�ݳ�
��ֹ������̼�ݳ�
��������з�Ӧ�Ļ�ѧ����ʽΪNa2CO3+2HCl�T2NaCl+H2O+CO2��
Na2CO3+2HCl�T2NaCl+H2O+CO2��
������NaOH��Һ��Ŀ�������ն�����̼
���ն�����̼
����4������ܹ��������Ŀ����
�����ɵĶ�����̼ȫ������װ��C��
�����ɵĶ�����̼ȫ������װ��C��
����5�����������ṩ�����ݼ����Ʒ��̼��������CO2�ĺ���Ϊ
20��y-x��
20��y-x��
g/L����6����û��Bװ�ã�����CO2������
ƫ��
ƫ��
�����ƫ��ƫС����ʵ�鷽������
�٢��뷽��һ��ͬ���۰�ͼ2����ʵ��װ�ã��Ƶ�����װ�ã���ҩƷ��������������ע������ϡ���ᣬ���10s������������װ�õ�������ֱ������װ�õ���������Ϊֹ��
��1��ע������ϡ��������Ļ�ѧ��Ӧ����ʽΪ
Na2CO3+2HCl�T2NaCl+H2O+CO2��
Na2CO3+2HCl�T2NaCl+H2O+CO2��
����2����ʵ���г����������ٽ���
3
3
�Σ���3��װ��B��Ŀ����
���������е�ˮ��
���������е�ˮ��
����4����ͬѧ����÷������CO2������ƫС����ͬѧ�����ɳ��˿����ǿ�����ˮ������ʵ������Ӱ���⣬��������
��ƿ�ڲ����ж�����̼
��ƿ�ڲ����ж�����̼
����������1�����ݳ������������ƽ��н��
��2�����ݿ�����ˮ�Ͷ�����̼��ʵ�������Ӱ����н��
��3�����ݶ�����̼���ܽ�����¶ȵĽ��Ͷ����������̼���Ʒ�Ӧ�����Ȼ��ơ�ˮ�Ͷ�����̼�Լ�����������Һ�����ն�����̼������н��
��4�����ݹ��������Ŀ���������ɵĶ�����̼ȫ������װ��C�н��н��
��5������Cװ���������Ӿ��Ƕ�����̼���������н��
��6������û��Bװ�ÿ����еĶ�����̼��ˮ�־ͻ���룬ʹ�ò��CO2������ƫ����н��
ʵ�鷽��������1�����������̼���Ʒ�Ӧ�����Ȼ��ơ�ˮ�Ͷ�����̼���н��
��2������ʵ���г����������н��
��3������Ũ������������Խ��н��
��4��������ƿ�ڲ����ж�����̼���н��
��2�����ݿ�����ˮ�Ͷ�����̼��ʵ�������Ӱ����н��
��3�����ݶ�����̼���ܽ�����¶ȵĽ��Ͷ����������̼���Ʒ�Ӧ�����Ȼ��ơ�ˮ�Ͷ�����̼�Լ�����������Һ�����ն�����̼������н��
��4�����ݹ��������Ŀ���������ɵĶ�����̼ȫ������װ��C�н��н��
��5������Cװ���������Ӿ��Ƕ�����̼���������н��
��6������û��Bװ�ÿ����еĶ�����̼��ˮ�־ͻ���룬ʹ�ò��CO2������ƫ����н��
ʵ�鷽��������1�����������̼���Ʒ�Ӧ�����Ȼ��ơ�ˮ�Ͷ�����̼���н��
��2������ʵ���г����������н��
��3������Ũ������������Խ��н��
��4��������ƿ�ڲ����ж�����̼���н��
����⣺��1�������ٵ�����Ϊ��Һ©���������ڵ�����Ϊ��ƿ��
��2��������ˮ�Ͷ�����̼��ʵ�������Ӱ�죬����Aװ�õ����������տ����еĶ�����̼��ˮ��Dװ�õ������Ƿ�ֹ�����еĶ�����̼��ˮ���룬Ӱ��ʵ������
��3��������̼���ܽ�����¶ȵĽ��Ͷ���������䶳̼�����ϵ�Ŀ���Ƿ�ֹ������̼�ݳ��������̼���Ʒ�Ӧ�����Ȼ��ơ�ˮ�Ͷ�����̼����ѧ��Ӧ����ʽΪNa2CO3+2HCl�T2NaCl+H2O+CO2��������������Һ�����ն�����̼���壬����NaOH��Һ��Ŀ�������ն�����̼��
��4�����������Ŀ���������ɵĶ�����̼ȫ������װ��C�У�
��5��Cװ���������Ӿ��Ƕ�����̼��������
���ɶ�����̼������=yg-xg
��Ʒ��̼��������CO2�ĺ���=
=20��y-x��g/L
�𣺴�Ʒ��̼��������CO2�ĺ���Ϊ20��y-x��g/L��
��6��û��Bװ�ÿ����еĶ�����̼��ˮ�־ͻ���룬ʹ�ò��CO2������ƫ��
ʵ�鷽��������1�������̼���Ʒ�Ӧ�����Ȼ��ơ�ˮ�Ͷ�����̼����ѧ��Ӧ����ʽΪNa2CO3+2HCl�T2NaCl+H2O+CO2����
��2����ʵ���г����������ٽ���3�Σ�
��3��Ũ������������ԣ����������е�ˮ�֣�
��4����ƿ�ڲ����ж�����̼�����CO2������ƫС��ʹ�ã�
�ʴ�Ϊ����1����Һ©������ƿ��
��2�����տ����еĶ�����̼��ˮ����ֹ�����еĶ�����̼��ˮ���룬Ӱ��ʵ������
��3����ֹ������̼�ݳ���Na2CO3+2HCl�T2NaCl+H2O+CO2�������ն�����̼��
��4�������ɵĶ�����̼ȫ������װ��C�У�
��5��20��y-x����
��6��ƫ��
ʵ�鷽��������1��Na2CO3+2HCl�T2NaCl+H2O+CO2������2��3����3�����������е�ˮ�֣���4����ƿ�ڲ����ж�����̼��
��2��������ˮ�Ͷ�����̼��ʵ�������Ӱ�죬����Aװ�õ����������տ����еĶ�����̼��ˮ��Dװ�õ������Ƿ�ֹ�����еĶ�����̼��ˮ���룬Ӱ��ʵ������
��3��������̼���ܽ�����¶ȵĽ��Ͷ���������䶳̼�����ϵ�Ŀ���Ƿ�ֹ������̼�ݳ��������̼���Ʒ�Ӧ�����Ȼ��ơ�ˮ�Ͷ�����̼����ѧ��Ӧ����ʽΪNa2CO3+2HCl�T2NaCl+H2O+CO2��������������Һ�����ն�����̼���壬����NaOH��Һ��Ŀ�������ն�����̼��
��4�����������Ŀ���������ɵĶ�����̼ȫ������װ��C�У�
��5��Cװ���������Ӿ��Ƕ�����̼��������
���ɶ�����̼������=yg-xg
��Ʒ��̼��������CO2�ĺ���=
| yg-xg |
| 50mL |
�𣺴�Ʒ��̼��������CO2�ĺ���Ϊ20��y-x��g/L��
��6��û��Bװ�ÿ����еĶ�����̼��ˮ�־ͻ���룬ʹ�ò��CO2������ƫ��
ʵ�鷽��������1�������̼���Ʒ�Ӧ�����Ȼ��ơ�ˮ�Ͷ�����̼����ѧ��Ӧ����ʽΪNa2CO3+2HCl�T2NaCl+H2O+CO2����
��2����ʵ���г����������ٽ���3�Σ�
��3��Ũ������������ԣ����������е�ˮ�֣�
��4����ƿ�ڲ����ж�����̼�����CO2������ƫС��ʹ�ã�
�ʴ�Ϊ����1����Һ©������ƿ��
��2�����տ����еĶ�����̼��ˮ����ֹ�����еĶ�����̼��ˮ���룬Ӱ��ʵ������
��3����ֹ������̼�ݳ���Na2CO3+2HCl�T2NaCl+H2O+CO2�������ն�����̼��
��4�������ɵĶ�����̼ȫ������װ��C�У�
��5��20��y-x����
��6��ƫ��
ʵ�鷽��������1��Na2CO3+2HCl�T2NaCl+H2O+CO2������2��3����3�����������е�ˮ�֣���4����ƿ�ڲ����ж�����̼��
����������Ͷ���ѧ�Ļ�ѧ֪ʶ�����ʵ���ɡ�̼���ƺ�ϡ����ķ�Ӧ�����ݻ�ѧ����ʽ�ļ�������ʵĴ��Ƚ����˿��飬Ҫ����������Щ֪ʶ����д��ѧ����ʽһ��Ҫȷ����

��ϰ��ϵ�д�
 ���ٴ���������ѧϰ����ѧ�ں����ν�ϵ�д�
���ٴ���������ѧϰ����ѧ�ں����ν�ϵ�д�
�����Ŀ

