��Ŀ����
 2011��9��29�գ�����������F��T1���ػ�������й�ȫ�����Ƶ���Ŀ����������칬һ�š��������գ�Ϊ��̫�ս����й��ռ�վ���»�����
2011��9��29�գ�����������F��T1���ػ�������й�ȫ�����Ƶ���Ŀ����������칬һ�š��������գ�Ϊ��̫�ս����й��ռ�վ���»�������1���ڶ�̫�յ�̽�������У���ѧ�ҷ������������ʯ���д��ڹ�̬��H2O������Hg��NH3��CH4�����ʣ��������ڵ��ʵ���
Hg
Hg
���������������H2O
H2O
������������CH4
CH4
�������Hg����������������ʰ���H2O��NH3��CH4��˳�����У������е���������Ԫ�ص�����������������
��Ԫ�ص�����������������
����2�����Ƿ���ǰ����ѧ�ҽ��Ӵ�����������۵�����װ���������ڣ������ǽ���Ԥ�������ֻ����£����߾ͻ��Զ�չ�����ָ���ԭ������״�����ڴ������������ϵ������������B
B
A��������״���书�� B�����кܵ͵��۵�
C���������õ���չ�� D���������õĵ�����
��3�������������N2H4����ȼ�ϣ���������������N2O4����ȼ�������ﲻ��Դ��������Ⱦ��
�ٷ��䡰��ˡ��롰�칬���Ļ����ƫ�����£�C2H8N2����������������N2O4���������ƽ���������ȫ��Ӧ�Ļ�ѧ����ʽ��C2H8N2+2N2O4�T3X+2CO2+4H2O����X�Ļ�ѧʽΪ
A
A
A��N2 B��H2 C��O2 D��CO
�����������������¾���ת���ɶ���������NO2��N2O4�Ƚϣ���ͬ����
BE
BE
��A����Ԫ�صĻ��ϼۡ�������B��������ԭ�ӵĸ�������������������C�����Ԫ��
D�������е�Ԫ�ص�������������������������E����Է���������
��4�����˺����������ģ�����У��������¹�������ͼ��ʾ��
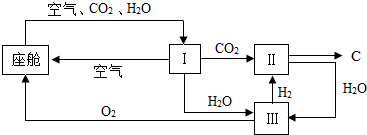
��װ�â��������
���������CO2��H2O
���������CO2��H2O
����װ�ã����е�CO2��H2�ڴ��������·����˷�Ӧ���䷴Ӧ�Ļ�ѧ����ʽΪ
CO2+2H2
C+2H2O
| ||
CO2+2H2
C+2H2O
��װ�â�����Ӧ�Ļ�ѧ����ʽΪ
| ||
2H2O
2H2��+O2����
| ||
2H2O
2H2��+O2����
��
| ||
�۴�װ�â�ɿ�����O2����Դ��CO2��H2O�����Աÿ������������к�1012gCO2��180gˮ��������ͨ����װ����ȫת����ɵõ�O2
896
896
g����������1���������ʷ�����й�֪ʶ�����жϣ�
��2�����ݲ��ϵ�Ӧ�û����ͷ����ĸı�����жϣ�
��3���ٸ��ݻ�ѧ�仯ǰ��ԭ�ӵ�����䡢ԭ�Ӹ���û���������ƶϷ�Ӧ���������ʵĻ�ѧʽ��
�ڸ��ݻ�ѧʽ��ʾ������������
��4���ٸ���װ��I�ṩˮ�Ͷ�����̼������
�ڸ���ͼʾ��֪��CO2��H2�ķ�Ӧ��������C��H2O��װ�â���ֻ��ˮ���ݴ�д����ѧ����ʽ���ɣ�
��ͨ��ͼʾ���Կ�����һװ��Ԫ���������仯�е�������û�иı䣬Ȼ�����û�ѧʽ�ļ��������⣮��
��2�����ݲ��ϵ�Ӧ�û����ͷ����ĸı�����жϣ�
��3���ٸ��ݻ�ѧ�仯ǰ��ԭ�ӵ�����䡢ԭ�Ӹ���û���������ƶϷ�Ӧ���������ʵĻ�ѧʽ��
�ڸ��ݻ�ѧʽ��ʾ������������
��4���ٸ���װ��I�ṩˮ�Ͷ�����̼������
�ڸ���ͼʾ��֪��CO2��H2�ķ�Ӧ��������C��H2O��װ�â���ֻ��ˮ���ݴ�д����ѧ����ʽ���ɣ�
��ͨ��ͼʾ���Կ�����һװ��Ԫ���������仯�е�������û�иı䣬Ȼ�����û�ѧʽ�ļ��������⣮��
����⣺��1���������ʵķ��࣬��������һ��Ԫ����ɵĴ���������ڵ��ʵ��� Hg��������������Ԫ������ұ��뺬����Ԫ�أ���������������� H2O���л�����Ҫ����̼Ԫ�أ��������л������ CH4�������Hg����������������ʰ���H2O��NH3��CH4��˳�����У��ӻ�ѧʽ������ص���������Ԫ�صĺ������ӣ���һ��Ԫ�صĺ������٣��������е���������Ԫ�ص�����������������
��2�����ݵ����ǽ���Ԥ�������ֻ����£����߾ͻ��Զ�չ�����ָ���ԭ������״�������ý���Ҫ�м��书�ܣ���Ҫ�����õ���չ�Ժ͵����ԣ�����۵�Ϳ��ܻ��ۻ�����ѡB��
��3�����ٸ��������غ㶨�ɣ���ѧ��Ӧǰ��ԭ�ӵĸ������䣬��Ӧǰ��Ӧ����N��H��OԪ�ص�ԭ�Ӹ�������Ϊ��6��8��4����Ӧ����������H��Oԭ�Ӹ���Ϊ8��4���ȽϷ�Ӧǰ��ԭ�����༰�����ɵ�֪��3��δ֪��ķ�����Ӧ����6��Nԭ�ӣ����ԣ�δ֪��Ļ�ѧʽΪN2��
�ڱȽ�NO2��N2O4�����ߵ�Ԫ����ɡ���Ԫ�صĻ��ϼۡ���Ԫ�ص�������������ͬ����ͬ���Ƿ�����ԭ�ӵĸ�������Է�����������ѡBE��
��3���ٸ���װ��I�ṩˮ�Ͷ�����̼��װ��I��Ϊ�˷��������ˮ�Ͷ�����̼��Ϊװ�â��ṩ������̼��ˮ��
����ͼ��֪��������ѧ�仯���Ǣ�ֱ͢��ǣ����з�����Ӧ�Ļ�ѧ����ʽΪ��CO2+2H2
C+2H2O��װ�â���ֻ��ˮ��ˮ���Ļ�ѧ����ʽΪ��2H2O
2H2��+O2����
��ͨ��ͼʾ���Կ�����һ���¹�������Ԫ���������仯�е�������û�иı䣬������������Ϊ��1012g��
+180g��
=896g
�ʴ�Ϊ����1��Hg��H2O��CH4����Ԫ�ص�����������������2��B
��3����A����BE��
��4���ٷ��������CO2��H2O����CO2+2H2
C+2H2O�� 2H2O
2H2��+O2������896g��
��2�����ݵ����ǽ���Ԥ�������ֻ����£����߾ͻ��Զ�չ�����ָ���ԭ������״�������ý���Ҫ�м��书�ܣ���Ҫ�����õ���չ�Ժ͵����ԣ�����۵�Ϳ��ܻ��ۻ�����ѡB��
��3�����ٸ��������غ㶨�ɣ���ѧ��Ӧǰ��ԭ�ӵĸ������䣬��Ӧǰ��Ӧ����N��H��OԪ�ص�ԭ�Ӹ�������Ϊ��6��8��4����Ӧ����������H��Oԭ�Ӹ���Ϊ8��4���ȽϷ�Ӧǰ��ԭ�����༰�����ɵ�֪��3��δ֪��ķ�����Ӧ����6��Nԭ�ӣ����ԣ�δ֪��Ļ�ѧʽΪN2��
�ڱȽ�NO2��N2O4�����ߵ�Ԫ����ɡ���Ԫ�صĻ��ϼۡ���Ԫ�ص�������������ͬ����ͬ���Ƿ�����ԭ�ӵĸ�������Է�����������ѡBE��
��3���ٸ���װ��I�ṩˮ�Ͷ�����̼��װ��I��Ϊ�˷��������ˮ�Ͷ�����̼��Ϊװ�â��ṩ������̼��ˮ��
����ͼ��֪��������ѧ�仯���Ǣ�ֱ͢��ǣ����з�����Ӧ�Ļ�ѧ����ʽΪ��CO2+2H2
| ||
| ||
��ͨ��ͼʾ���Կ�����һ���¹�������Ԫ���������仯�е�������û�иı䣬������������Ϊ��1012g��
| 32 |
| 44 |
| 16 |
| 18 |
�ʴ�Ϊ����1��Hg��H2O��CH4����Ԫ�ص�����������������2��B
��3����A����BE��
��4���ٷ��������CO2��H2O����CO2+2H2
| ||
| ||
������������Ҫ����ѧ��������ѧ��ѧ֪ʶ�ۺϷ����ͽ��ʵ�������������������ѧ�����������˼ά��ȣ�ǿ����ѧ������֪ʶ��������

��ϰ��ϵ�д�
�����Ŀ
 2011��9��29��21ʱ���칬һ�š�Ŀ��������ھ�Ȫ���Ƿ������ķ��䣨����ͼ��ʾ���������й����ռ�ʵ���ң����ֱ������۰˺š����۾źš�����ʮ�ŷɴ��Խӣ��Ӷ�������һ���й��ռ�ʵ���ң���ṹ��Ҫ�����ⲿ���塢ʵ��ա���Դ�ա�ȼ��ȼ��ϵͳ���Խӻ�����̫���ܵ��ȣ������������������µ�ʯī�ߡ�����֡��ѡ�����ͭ�ȵȣ��Իش��������⣺
2011��9��29��21ʱ���칬һ�š�Ŀ��������ھ�Ȫ���Ƿ������ķ��䣨����ͼ��ʾ���������й����ռ�ʵ���ң����ֱ������۰˺š����۾źš�����ʮ�ŷɴ��Խӣ��Ӷ�������һ���й��ռ�ʵ���ң���ṹ��Ҫ�����ⲿ���塢ʵ��ա���Դ�ա�ȼ��ȼ��ϵͳ���Խӻ�����̫���ܵ��ȣ������������������µ�ʯī�ߡ�����֡��ѡ�����ͭ�ȵȣ��Իش��������⣺ 2011��9��29��21ʱ16�֣��칬һ�ųɹ����䣨��ͼ��ʾ�������̫��ӭ����һ�����й����������䡰�칬һ�š����ػ��ʹ�õ�ȼ����ƫ������[��CH3��2NNH2]����������������������N2O4��Ϊ���������ʷ�Ӧ�������������岢�ͷų��������ȣ��÷�Ӧ�Ļ�ѧ����ʽΪ��2N2O4+��CH3��2NNH2�T3N2��+2CO2��+4H2O���Լ��㣺
2011��9��29��21ʱ16�֣��칬һ�ųɹ����䣨��ͼ��ʾ�������̫��ӭ����һ�����й����������䡰�칬һ�š����ػ��ʹ�õ�ȼ����ƫ������[��CH3��2NNH2]����������������������N2O4��Ϊ���������ʷ�Ӧ�������������岢�ͷų��������ȣ��÷�Ӧ�Ļ�ѧ����ʽΪ��2N2O4+��CH3��2NNH2�T3N2��+2CO2��+4H2O���Լ��㣺