��Ŀ����
�ᡢ����ڹ�ũҵ������������Ӧ�ù㷺����ش�������⣺
(1)��������ᶼ����Ҫ���ᣬ���Ǿ������ƻ�ѧ���ʵ�ԭ������ˮ��Һ�ж��ܽ����������Ӻ�_____(��д����)�������ڹ�ҵ�ϳ����ڽ������⣬д����һ����Ӧ�Ļ�ѧ����ʽ______��
(2)��ͼ���кͷ�Ӧ��ʵ����Ӧ�ù㷺������ijУ��ѧʵ���ҷ�Һ�����ԣ�Ӧѡ��________�Լ�(ָʾ��)����÷�Һ����ֱ���ŷŻᵼ�»�����Ⱦ���Ӿ��ÿ��еĽǶȿ��ǣ�ѡ��________�����÷�Һ��
(3)С�մ��DZ��Ƹ�����õķ��ͷ۵���Ҫ�ɷ�֮һ����ҽ���ϣ���������θ�����֢��һ��ҩ������д���÷�Ӧ�Ļ�ѧ����ʽ_______��
(4)���� Ba(NO3)2��Һ��ϡ���ᡢNa2CO3��Һ��KOH��Һ��CuSO4��Һ���������ѡ����ѡ���ܹ����������ʷ������ֽⷴӦ�����г������ɵ����ʣ�д���÷�Ӧ�Ļ�ѧ����ʽ_______��
A ϡ���� B �ռ���Һ C �������Һ
��ϰ��ϵ�д�
�����Ŀ


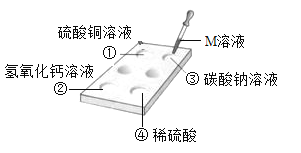
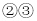 ��Һ��죬
��Һ��죬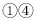 �ޱ仯����M����Ϊ��̪��Һ
�ޱ仯����M����Ϊ��̪��Һ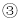 ��Ӧ������������M����Ϊϡ����
��Ӧ������������M����Ϊϡ����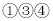 ���г������ɣ���M����Ϊ����������Һ
���г������ɣ���M����Ϊ����������Һ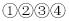 ��û��������M����Ϊ����������Һ
��û��������M����Ϊ����������Һ ��Һ C.NaOH��Һ D.
��Һ C.NaOH��Һ D. ��Һ
��Һ