��Ŀ����
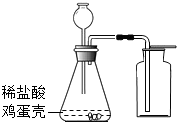
����ի��ѧ������ij��Ա������ͼװ��̽����ȡ����![]() ��ԭ�������������ʡ����װ��ͼ���ش��������⣺
��ԭ�������������ʡ����װ��ͼ���ش��������⣺
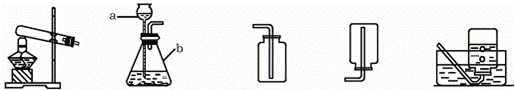
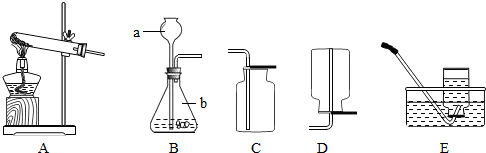
A B C D E
��1��д��ͼ�б���Сд��ĸ�����������ƣ� a ��
��2��ʵ���ҳ��ù���������Һ�Ͷ���������ȡ������
�����ѡ�õ���ȡO2��װ��Ҳ��������ȡCO2�������װ���� ��������ĸ��ţ�
��MnO2����������Ӧʹ�ã��ڸ÷�Ӧ���� ���á�
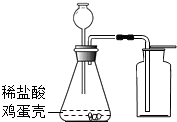
��3�������ǵ���Ҫ�ɷ���̼��ƣ��ü�������ϡ���ᷴӦ��ȡ���ռ�������̼���壬�䷴Ӧ�Ļ�ѧ����ʽ�� ��
��4���������ͼװ����ȡ������̼���壬��Ӧ����һ��ʱ���ȼ�ŵ�ľ�����ڼ���ƿ�ڣ����治Ϩ��Ŀ���ԭ���ǣ�
��
��5��ʵ������н������ǵ����Ŀ���ǣ�
��
��6����ʵ�����Ȼ�粒������ʯ�ҹ��干������ȡ������NH3����������NH3��һ����ɫ���д̼�����ζ�����壬�ܶȱȿ���С������ˮ��ʼ��ԡ���Ӧ�Ļ�ѧ����ʽΪ��
![]()
����ȡ���ռ�NH3��Ӧ�ô���ͼ��ѡ����ռ�װ���� ��
�ڷ�Ӧ������ֹͣ����ʱ������ �� ��
�۸���NH3ʱ����ѡ�����и�����е� ��������ţ�
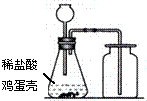 A�������������� B��Ũ���� C����ʯ��
A�������������� B��Ũ���� C����ʯ��
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�

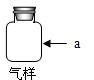
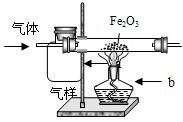
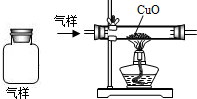 �ӱ�����ij������ú���ܵ�������COй©��һ��ѧ��ȤС���Ա�����ɼ��ó������������������������Ƿ���CO���壮С����һ��Ա���Ϸ�����ߣ���������ռ���һ����ƿ������������������ͼװ��������⣮
�ӱ�����ij������ú���ܵ�������COй©��һ��ѧ��ȤС���Ա�����ɼ��ó������������������������Ƿ���CO���壮С����һ��Ա���Ϸ�����ߣ���������ռ���һ����ƿ������������������ͼװ��������⣮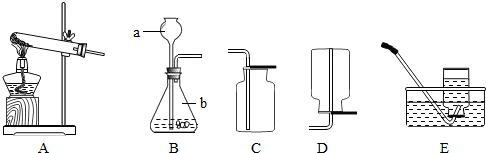
 CaCl2+2NH3��+2H2O
CaCl2+2NH3��+2H2O