��Ŀ����
��10̩��27��.��9�֣�ͨ����ѧѧϰ�����Ѿ�������ʵ������ȡ�����һ����ɣ���������ʦ�ṩ��һЩʵ��װ�á�

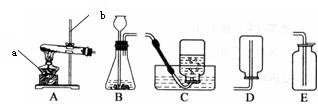
������ͼ�����ش����⣺
��1��д��ͼ�б�����������ƣ�a ��b ��
��2��д��ʵ������Aװ����ȡ�����Ļ�ѧ����ʽ ��
��3��ͨ���������ϵ�֪���ٰ�����NH3����һ���ܶȱȿ���С�Ҽ�������ˮ�����壬��ˮ��Һ��Ϊ��ˮ���Լ��ԣ��ڰ����ڼ�����������������ͭ��Ӧ����ͭ��ˮ�Ϳ����к����������塣С��ͬѧ�������Ȼ�狀��������ƵĹ���������ȡ��������Ӧ��ѡ��ķ�Ӧ����װ���� ���ռ�װ���� ������ĸ��ţ���
��4��С�����ռ��������ļ���ƿ�����ڵ�����ɫ��̪��ˮ�У��۲쵽��������
��
��5����д������������ͭ�ڼ��������·�Ӧ�Ļ�ѧ����ʽ ��

������ͼ�����ش����⣺
��1��д��ͼ�б�����������ƣ�a ��b ��
��2��д��ʵ������Aװ����ȡ�����Ļ�ѧ����ʽ ��
��3��ͨ���������ϵ�֪���ٰ�����NH3����һ���ܶȱȿ���С�Ҽ�������ˮ�����壬��ˮ��Һ��Ϊ��ˮ���Լ��ԣ��ڰ����ڼ�����������������ͭ��Ӧ����ͭ��ˮ�Ϳ����к����������塣С��ͬѧ�������Ȼ�狀��������ƵĹ���������ȡ��������Ӧ��ѡ��ķ�Ӧ����װ���� ���ռ�װ���� ������ĸ��ţ���
��4��С�����ռ��������ļ���ƿ�����ڵ�����ɫ��̪��ˮ�У��۲쵽��������
��
��5����д������������ͭ�ڼ��������·�Ӧ�Ļ�ѧ����ʽ ��
��1����Һ©�� �Թ�
��2��2H2O2 2H2O��O2��
2H2O��O2��
��3��B E
��4������ƿ��Һ������ ��Һ��Ϊ��ɫ
��5��3CuO��2NH3 3Cu��N2��3H2O
3Cu��N2��3H2O
��2��2H2O2
 2H2O��O2��
2H2O��O2����3��B E
��4������ƿ��Һ������ ��Һ��Ϊ��ɫ
��5��3CuO��2NH3
 3Cu��N2��3H2O
3Cu��N2��3H2O����1�����ݳ��������ʣ�1���𰸣���Һ©����1�֣��Թܣ�1�֣�
��2��˫��ˮ�ڶ������̵Ĵ�������Ѹ�ٷֽ�����ˮ�������ʣ�2���𰸣�2H2O2 2H2O+O2����1�֣�
2H2O+O2����1�֣�
��3����Ϊ�����Ȼ�狀��������ƵĹ���������ȡ������������NH3����һ���ܶȱȿ���С�Ҽ�������ˮ������ʣ�3���𰸣�B��1�֣�E��1�֣�
��4��������NH3����һ���ܶȱȿ���С�Ҽ�������ˮ�����壬��ˮ��Һ��Ϊ��ˮ���Լ��ԣ�װ�а����ļ���ƿ�����ڵ����̪��ˮ�۰�����������ˮ��ƿ����ѹ��С���ʴ𰸣�����ƿ��Һ��������1�֣���Һ��Ϊ��ɫ��1�֣�
��5�������ڼ�����������������ͭ��Ӧ����ͭ��ˮ�Ϳ����к����������壬�ʣ�5���𰸣�3CuO+2NH3 3Cu+N2+3H2O��2�֣�
3Cu+N2+3H2O��2�֣�
��2��˫��ˮ�ڶ������̵Ĵ�������Ѹ�ٷֽ�����ˮ�������ʣ�2���𰸣�2H2O2
 2H2O+O2����1�֣�
2H2O+O2����1�֣���3����Ϊ�����Ȼ�狀��������ƵĹ���������ȡ������������NH3����һ���ܶȱȿ���С�Ҽ�������ˮ������ʣ�3���𰸣�B��1�֣�E��1�֣�
��4��������NH3����һ���ܶȱȿ���С�Ҽ�������ˮ�����壬��ˮ��Һ��Ϊ��ˮ���Լ��ԣ�װ�а����ļ���ƿ�����ڵ����̪��ˮ�۰�����������ˮ��ƿ����ѹ��С���ʴ𰸣�����ƿ��Һ��������1�֣���Һ��Ϊ��ɫ��1�֣�
��5�������ڼ�����������������ͭ��Ӧ����ͭ��ˮ�Ϳ����к����������壬�ʣ�5���𰸣�3CuO+2NH3
 3Cu+N2+3H2O��2�֣�
3Cu+N2+3H2O��2�֣�
��ϰ��ϵ�д�
�����Ŀ






 CaCl2 + 2NH3��+ 2X��
CaCl2 + 2NH3��+ 2X��