��Ŀ����
��10Ϋ��24����(10��)̼������(NaHCO3)�׳�С�մ���һ�ְ�ɫ���壬�DZ��Ƹ�����õķ��ͷ۵���Ҫ�ɷ�֮һ��������ϡ������ᷴӦ����CO2���Իش�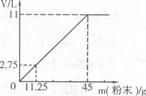
(1) д��NaHCO3��ϡ���ᷴӦ�Ļ�ѧ����ʽ ��
(2)�����98��������(�ܶ�Ϊ1��849��mL)����980918��4����������Һ?
��
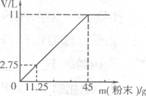
(3)�ֽ�45g NaHCO3(����KHCO3)�����ĩ����100mLϡ���ᣬǡ����ȫ��Ӧ��ʹ����ȫ���ݳ��������ĩ�����������CO2����Ĺ�ϵ��ͼ(��״���£�CO2���ܶ�Ϊ2g��L)��ͨ�����㣺
����100mLϡ�����������������
����ϡ����Ϊ120mLʱ����������ĩΪ58.5g�������CO2�������
(10��)
(1)2NaHCO3+H2SO4=Na2SO4+2CO2��+2H2O(2��)
(2)��100mL98����H2SO4�����ձ��ڱ���������796mLˮ�У�ͬʱ�ò��������Ͻ��衣(�÷�Ҫ�㣺100mLŨ�����1�֣�796mLˮ��1�֣�ŨH2SO4����ˮ�е�1�֣���3��)
(3)�⣺��m(CO2) ==11L��2g��L="22" g(1��)
��������Һ��H2SO4������Ϊx��
��(1)ʽ�ã�H2SO4 ~ 2CO2
98 88
x 22g
x=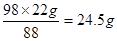 (2��)
(2��)
����120mLϡH2SO4��ȫ��Ӧ�Ĺ����ĩ������Ϊy�� y=54g<58.5g
y=54g<58.5g
�����ĩ������������������м��㣺
V(CO2)== (2��)
(2��)
��(��)
����

��1��ÿ�������ʴ��ɾ����ʧ������Ʒ��ʴ����Ҫԭ����
��2��У������ȤС��ȡΫ��ij������������Ʒ����ʵ�飺���ķݲ�ͬ������������Ʒ���ٶ�����ֻ���������͵���̼���ֱ�ӵ�100g����������ͬ��������Һ�У���ַ�Ӧ��õ�ʵ���������±���ʾ������֪���ڱ�״���£�22.4L H2������Ϊ2g��
| ʵ����� | 1 | 2 | 3 | 4 |
| ������Ʒ������/g | 2.88 | 5.76 | 9.24 | 10.28 |
| ����H2���������״���£�/L | 1.12 | 2.24 | 3.36 | m |
������������m��ֵΪ
�ڸ��ݱ������ݼ���������Һ��H2SO4������������
������������ʹ����������������������һ����Ҫ��־��
(1)ÿ�������ʴ������ɾ����ʧ������Ʒ��ʴ����Ҫԭ����__________
___________________________________________________________________��
 (2)У������ȤС���ͬѧ��ȡΫ��ij������������Ʒ����ʵ�飺���ķݲ�ͬ����
(2)У������ȤС���ͬѧ��ȡΫ��ij������������Ʒ����ʵ�飺���ķݲ�ͬ���� ��������Ʒ(�ٶ�����
��������Ʒ(�ٶ����� ֻ���������͵���̼)�ֱ�ӵ�100 g����������ͬ��ϡ�����У���ַ�Ӧ��õ�ʵ���������±�(��֪���ڱ�״���£�22.4 L H2������Ϊ2 g)
ֻ���������͵���̼)�ֱ�ӵ�100 g����������ͬ��ϡ�����У���ַ�Ӧ��õ�ʵ���������±�(��֪���ڱ�״���£�22.4 L H2������Ϊ2 g)
| ʵ����� | 1 | 2 | 3 | 4 |
| ������Ʒ������/g | 2.88 | 5.76 | 9.24 | 10.28 |
| ����H2����� (��״����)/L | 1.12 | 2.24 | 3.36 | m |
ͨ������ش��������⣺
������������m��ֵΪ_______________________________________________��
�ڸ��ݱ������ݼ���ϡ������H2SO4������������