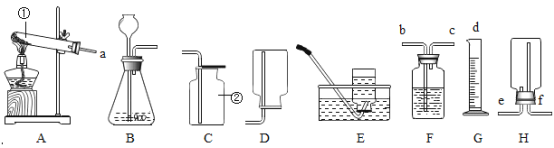
��Ŀ����
����Ŀ��С��ͬѧȥ��ɽ����ʱ,��ƿװ��һЩɽ�µ�Ȫˮ,����ʵ����,����ʦ��ָ�� ��,���������̽���ʵ��,��ȡ����ˮ����ش��������⣺
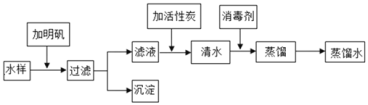
��1��ȡˮ�����������������_____��
��2�����й��˲���ʱ,���������������_____��
A ������Ҫ����������ֽ��һ��
B ©���¶˵Ĺܿ�Ҫ�����ձ����ڱ�
C ��ֽ�ı�ԵҪ����©����
D Һ�治Ҫ������ֽ��Ե
��3������Һ�м������̿,������_____��,��ȥˮ���е�ɫ�غ���ζ��
��4����������ˮ�ľ����̶��Ѿ��ܸ�,�����ǻ���ϰ������к�����,���˽�һ��ɱ������ˮ���д�����ϸ������,����Ϊ����ʲôԭ����_____��(���һ�㼴��)
��5�����̽��ˮ�����,�ɽ���ˮ�����,��д���÷�Ӧ�ķ��ű���ʽ_____��
���𰸡�������������ˮ�����ɵĽ�״������ʵ�����,ʹ������ˮ�е����ʳ��� D ���� ������ˮ��Ӳ�� ![]()
��������
��1�����������ھ�ˮ�е����ý��з������
��2������Һ��ʱ��ע�⡰һ�������͡���������ԭ��
��3������̿�����������ã��ܹ�����ˮ�е�ɫ�غ���ζ��
��4��������е����ý��з�����
��5�����̽��ˮ����ɣ��ɽ���ˮ���������д���÷�Ӧ�ķ��ű���ʽ��
��1�������dz��õľ�ˮ������������������ˮ�����ɵĽ�״������ʵ�������ʹ������ˮ�е����ʳ��������������������ˮ�����ɵĽ�״������ʵ�������ʹ������ˮ�е����ʳ�����
��2��A��������Ҫ����������ֽ��һ�ߣ���ѡ��˵����ȷ�����������⣻
B��©���¶˵Ĺܿ�Ҫ�����ձ����ڱڣ���ѡ��˵����ȷ�����������⣻
C����ֽ�ı�ԵҪ����©���ڣ���ѡ��˵����ȷ�����������⣻
D��Һ��Ҫ������ֽ��Ե����ѡ��˵������ȷ���������⡣
��ѡD��
��3������Һ�м������̿��Ŀ���ǣ�����̿���������ԣ�������ˮ�е�ɫ�غ���ζ�����������
��4����������ˮ��к����ã����˽�һ��ɱ������ˮ���д�����ϸ�����⣬��������ˮ��Ӳ�ȣ����������ˮ��Ӳ�ȣ�
��5����������̽��ˮ����ɣ��ɽ���ˮ�������ˮ���ʱ�����������������÷�Ӧ�ķ��ű���ʽ��![]() ���ʴ�Ϊ
���ʴ�Ϊ![]() ��
��

 �ʰ�Ӣ��ͬ����ϰ��ϵ�д�
�ʰ�Ӣ��ͬ����ϰ��ϵ�д� ѧϰʵ����ϵ�д�
ѧϰʵ����ϵ�д�����Ŀ��С��ͬѧ�ֱ��������ſ���������ˮ�����ռ�һƿ������Ȼ�������˿ȼ�յ�ʵ�飬����ȼ������ͬ�����Ƕ���˿ȼ��������Ũ�ȵĹ�ϵ������̽����
������ʵ�飩ȡ5����ͬ����˿��ֱ��0.6mm����������ͬ������״�ֱ����������������ͬ����ƿ�н���ʵ�飬ʵ���¼����
ʵ����� | �������������% | ʵ������ |
��һ�� | 90% | ȼ�վ��ң��������䣬ȼ��ʱ�䳤��ʵ��ɹ� |
�ڶ��� | 80% | ȼ��������90%�����û�����Բ��죬ʵ��ɹ� |
������ | 70% | ȼ�ձ�80%������ȼ��ʱ���80%�Ķ̣�ʵ��ɹ� |
���Ĵ� | 60% | ȼ�ձ�70%������ȼ��ʱ����̣�ʵ��ɹ� |
����� | 50% | ��˿û��ȼ�� |
��1����˿ȼ�յ����ֱ���ʽΪ_________
��2��ͨ������̽��ʵ�飬�ɵó��Ľ���________��
��3��Ҫ�о���˿�Ĵ�ϸ��ȼ�������Ӱ�죬����ʵ���ܴﵽĿ����_______��
A ��ͬһƿ�����У��Ⱥ���в�ͬ�֡�ϸ��˿��ȼ��ʵ��
B ����ƿ��ͬŨ�ȵ������У��ֱ�ͬʱ���д֡�ϸ��˿��ȼ��ʵ��
C ����ƿ��ͬŨ�ȵ������У��ֱ���д֡�ϸ��˿��ȼ��ʵ��
����˼�����ۣ�����������Ҫ��ϸߵ�ʵ��Ӧ��ȡ_____�ռ�������
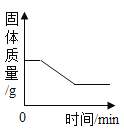
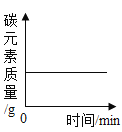
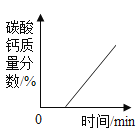
����Ŀ��ͬѧ����ѧϰ�����������ʺ�,֪������������ʹ�����ǵ�ľ����ȼ��,�ڴ˻�����,ͬѧ���������������,����һ������̽����
��1��������һ�������ǵ�ľ����ȼ�ܷ�֤�������Ǵ�����
������ʵ�飩ͬѧ�������ֻ�ʵ��̽���ǽ���ʵ��![]() ��ͼ
��ͼ![]() ,�������һϵ�����ݡ�
,�������һϵ�����ݡ�
����ƿ��� | �� | �� | �� | �� | �� |
����Ũ�ȣ���������� | 25% | 35% | 45% | �� | 65% |
������ľ����� | �� | �� | ���� | ��ȼ | ��ȼ |
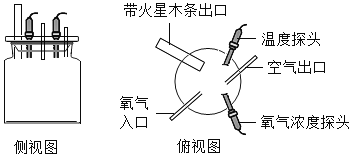
���ռ�֤�ݣ��ܺż���ƿ�ڵ�����Ũ�ȿ�����_____��
��ʵ����ۣ�_____��
����˼�����ۣ������������ݿ�֪���ռ�ƿ��Ϊ������������õ��ռ�������_____��
��2�������������˿�������е�ȼ��������Ũ�Ⱥ���˿��ϸ�й�ϵ��
ʵ���� | �� | �� | �� | �� | �� |
����Ũ�ȣ���������� | 34% | 47% | 60% | 73% | 86% |
ֱ��0.2mm��ϸ��˿ | ��ȼ�� | ����ȼ�� | ����ȼ�� | ����ȼ�� | ����ȼ�� |
ֱ��0.5mm�Ĵ���˿ | ��ȼ�� | ��ȼ�� | ��ȼ�� | ����ȼ�� | ����ȼ�� |
���ռ�֤�ݣ��ݺ�ʵ����ϸ��˿ȼ�յ�����ʵ��������_____��
��ʵ����ͣ��йط�Ӧ�Ļ�ѧ����ʽ��_____,ʵ�������,�ڼ���ƿ�ײ�������ˮ��Ŀ����_____��
��ʵ����ۣ���![]() ����Ũ��Խ��,��˿ȼ��Խ_____����_____��
����Ũ��Խ��,��˿ȼ��Խ_____����_____��
�������뽻�����������˿ȼ�յ�ʵ��ʱ,Ϊ��֤ʵ��ɹ���ע���һ��ʵ�������_____��
����Ŀ�����ճ������ũҵ�����У���Һ���Ź㷺��Ӧ�á�
I.��ũҵ��ͨ����12%���Ȼ�����Һѡ�֡�ʵ���������Ƹ�������������Һ60g����������ͼ����ش��������⣺
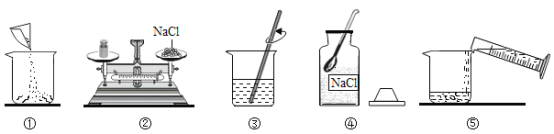
��1������ͼ�е���ű�ʾ������Һ����ȷ����˳��____________��
��2������۲�ڣ��������еĴ��������_______________���������и�������������������ȷ����������Һ�����ʵ���������_________��������������С����������������10%��
��3�����������õ��Ȼ�����Һ������������ƫС�����ܵ�ԭ����_________��
A ����Ͳ��ȡˮʱ���Ӷ���
B �ձ�������ˮ��ϴ��δ�����ɾ�������Һ
C ��ƽָ��ƫ�ҾͿ�ʼ����
D ת������õ���Һʱ����������Һ����
E ת���ѳƺõ��Ȼ��ƹ���ʱ�������������ձ���
II.����֪������ˮ�Ƽ�����ҵ�õ����DZ���ʳ��ˮ��ҽ����ʹ�õ�����0.9%��������ˮ���밴Ҫ����Ʋ��������ʵ�飺
ʵ����Ʒ���Ȼ�����Һ���Ȼ��ơ�����ء�����ˮ���ձ����Թܡ���������ҩ�ס�
̽��ʵ��һ�����������Ȼ�����Һ�Ƿ��DZ�����Һ
ʵ�鲽�� | ʵ������ | ʵ����� |
ȡһֻ�Թܵ���Լ5mL�Ȼ�����Һ����ҩ���������Ȼ��ƹ��壬���۲����� | �Ȼ��ƹ����ܽ� | _________ |
̽��ʵ��������������Ȼ�����Һ��Ϊ������Һ��̽�������Ȼ�����Һ���ܷ��ܽ�����ع���
ʵ�鲽�� | ʵ������ | ʵ����� |
_________ | _________ | NaCl��������Һ��Ϊ������Һ |
_________ | _________ | _________ |
������������