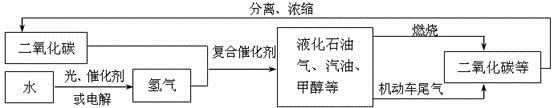
��Ŀ����
��Դ������������ȫ���ע���ȵ�����.
��1�����ǰ�ֱ�Ӵ���Ȼ��ȡ�õ���Դ��Ϊһ����Դ��һ����Դ�����ӹ�ȡ�õ���Դ��Ϊ������Դ��������Ŀǰ�������úͿ����IJ�����Դ����ú�������ͣ�����Ȼ������̫���ܣ��ݷ��ܣ���ϫ�ܣ��ߵ����ܣ�����ܣ������ܣ���ƾ������в�����һ����Դ���ǣ�����ţ� ��
��2��ú��ȼ�ջ���������Ķ���������ú�м���������ʯ��ʯ�����Ƴɻ���ú��ȼ��ʱʯ��ʯ�е�̼�����������������ڸ��µ���������������ƺͶ�����̼��д���÷�Ӧ�Ļ�ѧ����ʽ ��
��3������ͨ�����м���������ȼ���Ҵ����Ƶ��Ҵ����ͣ����Ҵ�������Ϊ����ȼ��Խ��Խ�ܵ����ӡ�������ʹ���Ҵ����͵��ŵ㣨��һ�����ɣ� ���Ҵ����ȼ�յĻ�ѧ����ʽΪ ��
��1���ڢ��⣻��2��2CaCO3+2SO2+O2 2CaSO4+2CO2����3����ʡʯ����Դ�����������β������Ⱦ����ٽ�ũҵ��������C2H5OH + 3O2
2CaSO4+2CO2����3����ʡʯ����Դ�����������β������Ⱦ����ٽ�ũҵ��������C2H5OH + 3O2 2CO2 + 3H2O
2CO2 + 3H2O
���������������1���������е���������֪������һ����Դ���Ǣڢ��⣻��2��ú��ȼ�ջ���������Ķ���������ú�м���������ʯ��ʯ�����Ƴɻ���ú��ȼ��ʱʯ��ʯ�е�̼�����������������ڸ��µ���������������ƺͶ�����̼��д���÷�Ӧ�Ļ�ѧ����ʽΪ��2CaCO3+2SO2+O2 2CaSO4+2CO2����3������ͨ�����м���������ȼ���Ҵ����Ƶ��Ҵ����ͣ����Ҵ�������Ϊ����ȼ��Խ��Խ�ܵ����ӡ�ʹ���Ҵ����͵��ŵ㣺��ʡʯ����Դ���Ҵ����ȼ�յĻ�ѧ����ʽΪC2H5OH + 3O2
2CaSO4+2CO2����3������ͨ�����м���������ȼ���Ҵ����Ƶ��Ҵ����ͣ����Ҵ�������Ϊ����ȼ��Խ��Խ�ܵ����ӡ�ʹ���Ҵ����͵��ŵ㣺��ʡʯ����Դ���Ҵ����ȼ�յĻ�ѧ����ʽΪC2H5OH + 3O2 2CO2 + 3H2O
2CO2 + 3H2O
���㣺��ѧ����Դ������

 �ݾ�ѵ������ϵ�д�
�ݾ�ѵ������ϵ�д� С����ȫ�ܼ��ϵ�д�
С����ȫ�ܼ��ϵ�д�| A���ƾ��ӷ� | B��������� | C��ʯ���ۻ� | D��ʪ������ |
A�� ����ȼ�� | B�� H2O2��Һ�м���MnO2 | C�� �������� | D�� ����̿�������� |
ijУ����ѧϰС��ļס��ҡ���������λͬѧ�ԡ�������Ϊ�����ѧ���ۡ���˵����������������Ͳ��ǻ�ѧ�о���������˵�������С�CNG����������Ȼ��Ϊȼ�ϵ�����������˵���������Ľ�����������Ŀ��ֻ�������۵����á�����˵��������β�����ŷŻ�Ի��������Ⱦ�����������ѧ֪ʶ�ж�˵�������ͬѧ��
| A����ͬѧ | B����ͬѧ | C����ͬѧ | D����ͬѧ |
��6�֣���ʡ��������ú̿��Դ�ḻ����˹������ú�㼰��Χ�Ҳ��У��Ǿ����к�������ܳƣ���Ҫ�ɷ��Ǽ��顣
��1������д��������ȫȼ�յĻ�ѧ����ʽ��__________________________________��
��2��ú����˹��ը��������Ҫ������
����˹�����ڱ�ը���ķ�Χ�ڣ���________________________________________��
��3���±��dz�����������ı�ը���ޣ�����ݴ��жϣ�
����������ը��������____��
| ��ȼ���� | ��ը���ޣ���������� |
| H2 | 4.0%~74.2% |
| CH4 | 5%~15% |
| CO | 12.5%~74.2% |
��4������ͼ���У���ȼ�պͱ�ը�ص���

A B C D
��5��ij�ִ����Ŀ����ˡ���Ũ����˹���缼��������Ч����˿������ܱߵ����������õ硣���ּȲ�ú�ַ��������������ŵ���___________________________________��
���й���ʯ�͵���������ȷ����
| A��ʯ����һ�ֻ�����Ʒ |
| B��ʯ����һ�ֻ���� |
| C��������ʯ�Ͳ�Ʒ���� |
| D����ʯ�ͷ���ɵõ����ֲ�Ʒ |
�������������ʣ������������γɵ�������ʯ�ͻ������ų��ķ����ۿ����е�ˮ������ֲ����й�����÷ų��������úȼ�ղ������̡�����ʹ����������Ⱦ����������ɺܴ�Σ����������
| A���ۢܡ��� | B���٢ڢݡ��� | C���ۢݡ��� | D���٢ۢ� |
 6H2O+�� ����
6H2O+�� ����