��Ŀ����
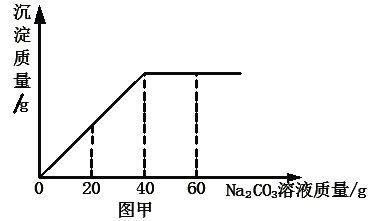
����Ŀ��ʵ������һƿδ֪Ũ�ȵ�BaCl2��Һ��ijͬѧȡ��150g����Һ���ձ��У���������μ���������������Ϊ26.5%��Na2CO3��Һ����Ӧ���������ɳ���������������Na2CO3��Һ�����Ĺ�ϵ��ͼ����ʾ����֪��BaCl2��Na2CO3��BaCO3����2NaCl ����㣺
��1������26.5%��Na2CO3��Һ80g����ҪNa2CO3���� g��
��2��BaCl2��Һ���������������Ƕ��٣���д��������̣����������0.1%��
���𰸡���1��21.2g ��2��13.9%
��������
�����������1�� ����26.5%��Na2CO3��Һ80g����ҪNa2CO3����Ϊ��80g��26.5%=21.2g
��2���⣺��ͼ���л�֪����BaCl2��ȫת��Ϊ����ʱ����Na2CO3��Һ������Ϊ40g��
��BaCl2������Ϊx����
BaCl2��Na2CO3��BaCO3����2NaCl
208 106
x 40g��26.5%
![]()
��ã�x��20.8g ��3�֣�
��BaCl2��Һ������������������20.8g/150g��x 100%=13.9%
�𣺸�BaCl2��Һ��������������Ϊ13.9%��

 ����ѵ�����⿼ϵ�д�
����ѵ�����⿼ϵ�д� �������ϵ�д�
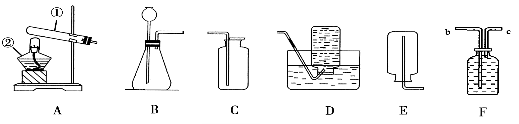
�������ϵ�д�����Ŀ��ˮ��ʵ��������Ҫ���ã�����ʵ����ˮ�����ý��ʹ������
A | B | C | D | |
ʵ �� �� �� |
�������� |
���������������IJⶨװ�� |
��������� |
�������� |
ˮ �� �� �� | ����ǰ�ò�����պˮ���������á� | ƿ�е�ˮֻ�弯��ƿ����ѹ����Ȼ�������� | �γ��ܱ�ϵͳ�����ڹ۲�������װ���Ƿ�©���� | ��ϴƿ������ˮ������ֽ����©���ڱڡ� |