��Ŀ����
ijѧϰС�����ʵ�������չ�������̼����ļ�ʯ�ҳɷֽ������о������������ϡ�
�ټ�ʯ����CaO��NaOH �Ĺ������ͨ���������ն�����̼���������壮
�ڼ��Ե�Na2CO3��Һ���������Ե�CaCl2��Һ�������ֽⷴӦ��
��������롿
�ü�ʯ�ҵ���Ҫ�ɷֿ��ܺ��� ��Ca��OH��2��CaCO3��NaOH�� ��
����Ʒ������ռ�֤�ݡ�
��1����ͬѧ���ձ��з��������ļ�ʯ����Ʒ��������������ˮ��ֽ��裬�����а�ɫ��������ͬѧ��Ϊ��Ʒ��һ������CaCO3����ͬѧ��Ϊ���Ľ��۲�ȷ��ԭ���ǣ� ���ѧ����ʽ����
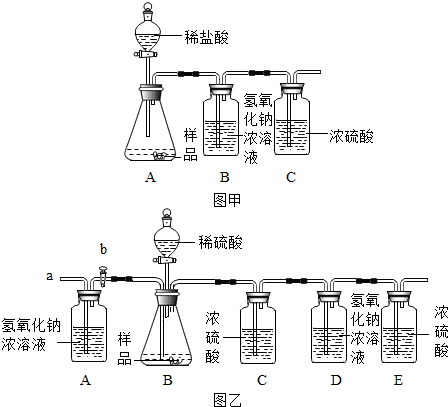
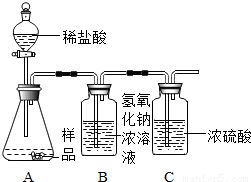
��2����ͬѧ��һ�����ʵ�鲢������֤���������£�
| ʵ����� | ʵ������ | ʵ����� |
| �ٴӼ�ͬѧ���ձ���ȡ�����ϲ���Һ���Թ��У������еμ����� ��Һ�� | �а�ɫ�������� | ���ɰ�ɫ�����Ļ�ѧ����ʽΪ�� �� |
| �ڹ��ˣ�����Һ�еμ���ɫ��̪��Һ�� | ���������� | ͨ��ʵ�鼰��������Ʒ�в����У� �� |
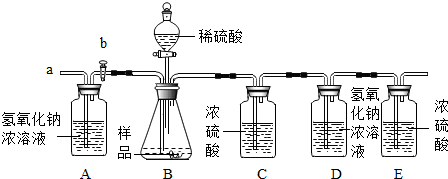
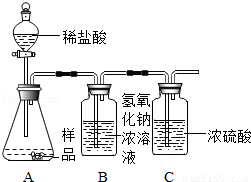
��1����ͬѧ��һ�����������ʵ��װ�ã�ͨ������Bװ�õ������仯��ȷ����Ʒ�ijɷּ����������������ã�ÿ������ȫ��Ӧ����������
���ʵ�鷢�ֲⶨ�����ʱƫ����ʱƫСƫ��Է�������Ҫԭ�� ��
��2������ʦ��ָ������ͬѧ������ʵ��װ�ã������½�����ʵ�飬�������£�
�ټ��װ�������ԣ���5.06g�������Ʒ������ƿ�У�
�ڴ���b���ӵ���a����������һ�����Ŀ������رջ���b��
�۳���ʢ����������Ũ��Һ��Dƿ������
������ƿ����μ���ϡ���������ٲ������ݣ�
�ݴ���b���ӵ���a����������һ�����Ŀ�����
����Dƿ����������2.2g��
ͨ�������������֪��5.06g��Ʒ�и��ɷּ����� ��
����Ʒ������ռ�֤�ݡ�
��1������̼�������������Ʒ�Ӧ�ܲ�����������
��2������̼���ƵĻ�ѧ���ʷ��������ݼ�����Һ��ʹ��̪��Һ������
�����������ۡ���1������װ���ص㡢ҩƷ���������������ԭ��
��2��������Ʒ�����������ɵĶ�����̼�����������ݻ�ѧ����ʽ�������
����⣺��������롿�ü�ʯ��������������ˮ��Ӧ�����������ƣ����������������̼��Ӧ����̼��ƺ�ˮ�������������������̼��Ӧ����̼���ƺ�ˮ�����ն�����̼�����Ҫ�ɷֿ��ܺ��к��� CaO��Ca��OH��2��CaCO3��NaOH�� Na2CO3
����Ʒ������ռ�֤�ݡ�
��1����ͬѧ���ձ��з��������ļ�ʯ����Ʒ��������������ˮ��ֽ��裬�����а�ɫ��������ͬѧ��Ϊ��Ʒ��һ������CaCO3����˵����ȷ����Ϊ��ʯ����Ʒ�п��ܺ���̼���ƺ��������ƣ�̼�������������Ʒ�Ӧ������̼��Ƴ�����
��ѧ����ʽΪ Na2CO3+Ca��OH��2=CaCO3��+2NaOH��
��2����ͬѧҪ��֤���Ƿ���̼���ƣ���̼�������Ȼ��Ʒ�Ӧ������̼��ư�ɫ��������˿ɴӼ�ͬѧ���ձ���ȡ�����ϲ���Һ���Թ��У������еμ����� CaCl2��Һ���а�ɫ�������ɣ�˵������̼���ƣ���ѧ����ʽΪNa2CO3+CaCl2=CaCO3��+2NaCl��Ȼ����ˣ�����Һ�еμ���ɫ��̪��Һ������������˵����Ʒ��һ�������м������ʣ����һ������Ca��OH��2��NaOH�� CaO��
�����������ۡ���1����ͬѧ���ʵ��װ�ã���Ʒ�м������ᣬͨ������Bװ������������Һ�������仯��ȷ�����ɵĶ�����̼���������Ӷ�ȷ����Ʒ�ijɷּ�����������ƿ�в�����CO2δ�����ջᵼ�½��ƫС�����ӷ��������Ȼ��⼰�����ж�����̼��ˮ������������ᵼ�½��ƫ��ÿ��ʵ�����������ۺ����ã�������CO2�������ڻӷ��������Ȼ��⼰�����ж�����̼ˮ�����������½��ƫС����֮����ƫ��
��2������Ʒ��̼��������Ϊx��̼�������Ϊ5.06g-x
Na2CO3+H2SO4�TNa2SO4+H2O+CO2��
106 44
x
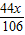
CaCO3+H2SO4�TCaSO4+H2O+CO2��
100 44
5.06g-x
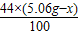
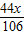 +
+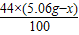 =2.2g
=2.2gx=1.06g
̼�������Ϊ5.06g-1.06g=4g
�ʴ�Ϊ����������롿
CaO Na2CO3
����Ʒ������ռ�֤�ݡ�
��1��Na2CO3+Ca��OH��2=CaCO3��+2NaOH
��2��
| CaCl2�� | Na2CO3+CaCl2=CaCO3��+2NaCl�� Ca��OH��2��NaOH�� CaO�� | |
��1����ƿ�в�����CO2δ�����ջᵼ�½��ƫС�����ӷ����������ἰ�����ж�����̼��ˮ������������ᵼ�½��ƫ��ÿ��ʵ�����������ۺ����ã�������CO2�������ڻӷ����������ἰ�����ж�����̼ˮ�����������½��ƫС����֮����ƫ��
��2��CaCO3��4�� Na2CO3��1.06g��
���������⿼���ʯ�Ҹ�������ʺ�IJ���ۺϿ����������ơ��������ơ��������ƵĻ�ѧ���ʣ��ۺ��Խ�ǿ

ijѧϰС�����ʵ�������չ�������̼����ļ�ʯ�ҳɷֽ������о���
���������ϡ�
�ټ�ʯ����CaO��NaOH �Ĺ������ͨ���������ն�����̼���������壮
�ڼ��Ե�Na2CO3��Һ���������Ե�CaCl2��Һ�������ֽⷴӦ��
��������롿
�ü�ʯ�ҵ���Ҫ�ɷֿ��ܺ����� ����Ca��OH��2��CaCO3��NaOH���� ����
����Ʒ������ռ�֤�ݡ�
��1����ͬѧ���ձ��з��������ļ�ʯ����Ʒ��������������ˮ��ֽ��裬�����а�ɫ��������ͬѧ��Ϊ��Ʒ��һ������CaCO3����ͬѧ��Ϊ���Ľ��۲�ȷ��ԭ���ǣ��� �����ѧ����ʽ����
��2����ͬѧ��һ�����ʵ�鲢������֤���������£�
|
ʵ����� |
ʵ������ |
ʵ����� |
|
�ٴӼ�ͬѧ���ձ���ȡ�����ϲ���Һ���Թ��У������еμ������� ����Һ�� |
�а�ɫ�������� |
���ɰ�ɫ�����Ļ�ѧ����ʽΪ�� �� ���� |
|
�ڹ��ˣ�����Һ�еμ���ɫ��̪��Һ�� |
���������� |
ͨ��ʵ�鼰��������Ʒ�в����У� �� ���� |
�����������ۡ�
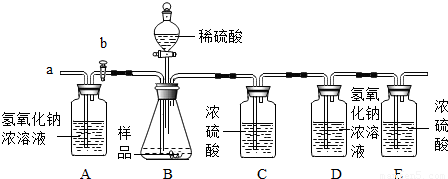
��1����ͬѧ��һ�����������ʵ��װ�ã�ͨ������Bװ�õ������仯��ȷ����Ʒ�ijɷּ����������������ã�ÿ������ȫ��Ӧ����������
���ʵ�鷢�ֲⶨ�����ʱƫ����ʱƫСƫ��Է�������Ҫԭ���� ��
��2������ʦ��ָ������ͬѧ������ʵ��װ�ã������½�����ʵ�飬�������£�

�ټ��װ�������ԣ���5.06g�������Ʒ������ƿ�У�
�ڴ���b���ӵ���a����������һ�����Ŀ������رջ���b��
�۳���ʢ����������Ũ��Һ��Dƿ������
������ƿ����μ���ϡ���������ٲ������ݣ�
�ݴ���b���ӵ���a����������һ�����Ŀ�����
����Dƿ����������2.2g��
ͨ�������������֪��5.06g��Ʒ�и��ɷּ������� ������
ijѧϰС�����ʵ�������չ�������̼����ļ�ʯ�ҳɷֽ������о���
���������ϡ�
�ټ�ʯ����CaO��NaOH �Ĺ������ͨ���������ն�����̼���������壮
�ڼ��Ե�Na2CO3��Һ���������Ե�CaCl2��Һ�������ֽⷴӦ��
��������롿
�ü�ʯ�ҵ���Ҫ�ɷֿ��ܺ�������Ca��OH��2��CaCO3��NaOH������
����Ʒ������ռ�֤�ݡ�
��1����ͬѧ���ձ��з��������ļ�ʯ����Ʒ��������������ˮ��ֽ��裬�����а�ɫ��������ͬѧ��Ϊ��Ʒ��һ������CaCO3����ͬѧ��Ϊ���Ľ��۲�ȷ��ԭ���ǣ���N�����ѧ����ʽ����
��2����ͬѧ��һ�����ʵ�鲢������֤���������£�
| ʵ����� | ʵ������ | ʵ����� |
| �ٴӼ�ͬѧ���ձ���ȡ�����ϲ���Һ���Թ��У������еμ�����������Һ�� | �а�ɫ�������� | ���ɰ�ɫ�����Ļ�ѧ����ʽΪ�� ������ |
| �ڹ��ˣ�����Һ�еμ���ɫ��̪��Һ�� | ���������� | ͨ��ʵ�鼰��������Ʒ�в����У� ������ |
�����������ۡ�
��1����ͬѧ��һ�����������ʵ��װ�ã�ͨ������Bװ�õ������仯��ȷ����Ʒ�ijɷּ����������������ã�ÿ������ȫ��Ӧ����������
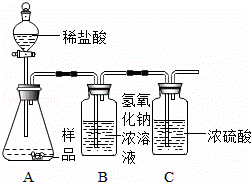
���ʵ�鷢�ֲⶨ�����ʱƫ����ʱƫСƫ��Է�������Ҫԭ��������
��2������ʦ��ָ������ͬѧ������ʵ��װ�ã������½�����ʵ�飬�������£�
�ټ��װ�������ԣ���5.06g�������Ʒ������ƿ�У�
�ڴ���b���ӵ���a����������һ�����Ŀ������رջ���b��
�۳���ʢ����������Ũ��Һ��Dƿ������
������ƿ����μ���ϡ���������ٲ������ݣ�
�ݴ���b���ӵ���a����������һ�����Ŀ�����
����Dƿ����������2.2g��
ͨ�������������֪��5.06g��Ʒ�и��ɷּ�����������