��Ŀ����
����Ŀ��������ճ������ũҵ�����벻��ˮ����ش�:
��1�����ˮʵ���ʾ��ˮ����ɣ���Ӧ�Ļ�ѧ����ʽΪ________________������ˮ�Ļ�ѧ���ʵ���С������____________������ţ�
��2��Ӳˮ����__________�������������������������������Ҫ���鱾����������ˮ�Ƿ�ΪӲˮ����ѡ�õ�������___________�������г���________��������ˮ��Ӳ�ȡ�
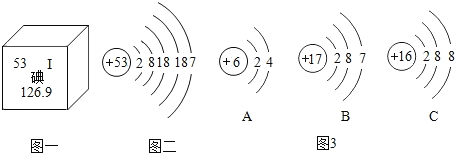
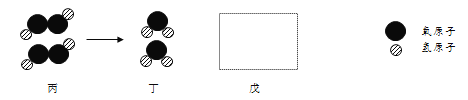
��3���Ӻ�ˮ��������������ˮ(��ѧʽΪD2O)������ԭ��(D)�����ԭ��������2������ˮ����Ԫ�ص���������Ϊ___________��
��4�����й���ˮ��˵����,����ȷ����_____
A ˮ����������������� B ����ʩ��ũҩ�����ʣ��Լ���ˮ����Ⱦ
C ����ˮ�������㣬����Ϊ����ˮ�в�����Ԫ�� D ϴ�ˡ�ϴ�º��ˮ������ϴ����
��5��ˮ��������Ϊ��һ����Ԫ����������ʵ����֤��ˮ�����⡢������Ԫ����ɵ�����_______��
A ������������ȼ�� B ˮ������ C ˮ�ĵ��
��6����ҵ����ˮ�ͼ��飨CH4��������һ�������·�Ӧ����һ����̼���������÷�Ӧ�Ļ�ѧ����ʽΪ_______________________��
���𰸡�2H2O![]() 2H2��+O2�� ˮ���� ����� ����ˮ ��� 80% AC AC H2O +CH4
2H2��+O2�� ˮ���� ����� ����ˮ ��� 80% AC AC H2O +CH4 CO+3H2
CO+3H2
��������
��1�����ˮ��������������������ʾ��ˮ����ɣ���Ӧ�Ļ�ѧ����ʽΪ��2H2O![]() 2H2��+O2����ˮ����ˮ���ӹ��ɣ�����ˮ�Ļ�ѧ���ʵ���С������ˮ���ӣ�
2H2��+O2����ˮ����ˮ���ӹ��ɣ�����ˮ�Ļ�ѧ���ʵ���С������ˮ���ӣ�
��2��Ӳˮ�к��иơ�þ���ӵĻ������ˮ�����ڻ�����Ȼˮ���������ˮ��������϶���ĭ������ˮ�����ٵ���ĭ��Ӳˮ�����Գ��÷���ˮ����Ӳˮ����ˮ������Ӳˮ�����ʼ������ʱ�ܹ�ת��Ϊ���������ʶ�����������ת��Ϊ��ˮ�����������п��� ������еķ�������ˮ��Ӳ�ȣ�
��3����ˮ����ѧʽΪD2O��������ԭ�ӣ�D�������ԭ��������2������ˮ����Ԫ�ص���������Ϊ![]() ��
��
��4��A ˮ�Ǵ����������������������ɣ���A����
B����ʩ��ũҩ�����ʣ��Լ���ˮ����Ⱦ����B��ȷ��
C����ˮ�������㣬����Ϊ����ˮ�в����������Ժ�����Ԫ�أ���C����
Dϴ�ˡ�ϴ�º��ˮ������ϴ�����ɽ�Լˮ��Դ����D��ȷ����ѡAC��
��5������������Ԫ����ɣ�����������Ԫ����ɣ��ڻ�ѧ��Ӧ��Ԫ�ص�����䣬������������ȼ������ˮ��ˮ�ĵ��ֽ�������������������֤��ˮ�����⡢������Ԫ����ɣ� ˮ�������������仯������֤��ˮ����ɡ���ѡAC��
��6����ҵ����ˮ�ͼ��飨CH4��������һ�������·�Ӧ����һ����̼���������÷�Ӧ�Ļ�ѧ����ʽΪH2O +CH4 CO+3H2
CO+3H2