��Ŀ����
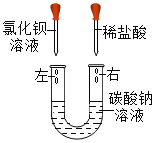
���һ����ܾ��ѹ涨������ʵ�������뻷����ܷ�Χ��ij��ѧ���꼶�ס����������ͬѧ�ֱ���������ͼ���ָʾ����Ӧ��ʵ�飬������ʵ���ʹ�õ��Լ���ͼ��ʾ��ʵ����Һ�ֱ��е�����ķ�Һ���У��װ��Һ�ʺ�ɫ���Ұ��Һ����ɫ��
��1�����������Һ�ijɷ֣��װ��Һ����_________���Ұ��Һ�к���________________��
��2��Ŀǰ�����У����ѧ�Լ����е�λ�Ļ�ѧʵ�����ŷŵĸ����Һ��δ���ϸ������������ˮ�����װ�ķ�Һ��ֱ��������������ˮ���������ʲôΣ��?________________���㽨����δ����װ�ķ�Һ?_____________________________��
 ����ѧҵ���Ե�����ϵ�д�
����ѧҵ���Ե�����ϵ�д������±��ش����⣺
�¶�/�� | 20 | 40 | 60 | |
�ܽ�� | NaCl | 36.0 | 36.6 | 37.3 |
KNO3 | 31.6 | 63.9 | 110 |
��1��60�棬100gˮ��������ܽ�KNO3������Ϊ_____��
��2������˵����ȷ����_____������ţ�
A 20��ʱ��KNO3������Һ����������Ϊ31.6%
B 40��ʱ��136.6gNaCl��Һ��һ������36.6gNaCl
C ��40��ʱKNO3�ı�����Һ���µ�20�棬����32.3gKNO3����
D ��40��ʱNaCl��KNO3�ı�����Һ�ֱ�����20�棬���º�����Һ���������Ĵ�С��ϵΪNaCl��KNO3
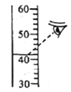
��3��20��ʱ��������ͼʾ������

������ҺA��B��C�У�KNO3�ܽ�ﵽ����״̬����_____������ĸ��
�ڱȽ���ҺA��B��KNO3������������A_____B�����������������
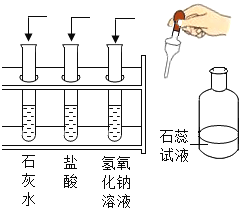
����������ѧ֪ʶ����ʹ��ͷ�Ը����������¶�Ӧ��ȫ��ȷ��һ����( )
A | ���ʵ���������; | B | ��ȫ��ʶ |
N2�����ȶ���������������� ʯī�����������缫 | �پ��ж����ɼ�ȩ���� ú��ը������˹���� | ||
C | Ԫ�������彡�� | D | �ճ������ |
ȱά����C��������Ѫ�� ȱ�ơ��������ɻ�����Ͳ� | ʳƷ�����������CuO ������ë��ά����ȼ��������ë��ζ |
A. A B. B C. C D. D


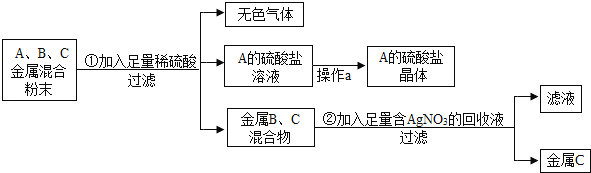
 B. ���װ�õ�������
B. ���װ�õ�������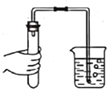
 D. �㵹Һ��
D. �㵹Һ��