��Ŀ����
����Ŀ����ͼ��ʾ���������������Һ������Ӧ��������Һ��pH�ı仯���ߡ��������ͼ�л�ȡ��Ϣ���ش��������⣺
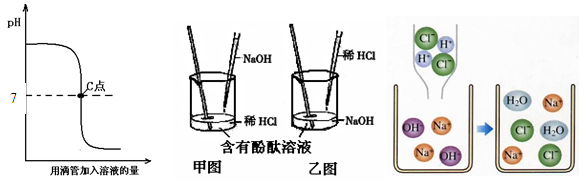
��1��ʵ������ǰ������� �����ң�ͼ��ʾ���������ձ�����Һ����ɫ�� ɫ��Ϊ ɫ��������Ӧ�Ļ�ѧ����ʽΪ ��
��2��ʵ�����ʱ����ʦ�μ����η�Ӧ�����Һ������Ƭ�ϣ���ɺ���ְ�ɫ���塣С��˵�����������ƣ�С����Ϊ�����ܣ�֤���� ��
��3������ͼ�Ƿ�Ӧ���۱仯ͼ���Աȷ�Ӧǰ��������˷�Ӧ��ʵ����������Һ�е� ������������Һ�е����������ӽ�ϣ�������ˮ����ͼ��������ܷ������Ʒ�Ӧ���볢����д���ᣨHClO3�����������أ�KOH����Ӧ�Ļ�ѧ����ʽ ��
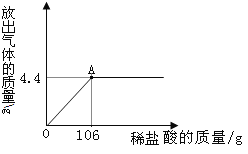
��4�������ʵ���������Ϊ36.5%������20g����������������Һǡ����ȫ��Ӧ����Ҫ���ʵ���������Ϊ20%������������Һ g��
���𰸡�(1)�ң�1�֣����죨1�֣����ޣ�1�֣���NaOH+HCl==NaCl +H2O��2�֣� (2)�����з�̪�ģ��ձ��е���Һ����ɫ��1�֣� (3)�����ӣ���H+����1�֣���KOH+ HClO3 ==KClO3 +H2O��2�֣� ��4��40��2�֣�
��������
�����������1����pHͼ���֪���ò���������������м����ᡣ����Ӧ����������ͼ��ʾ�����ġ���ʼʱ�ձ��е�����Ϊ�������ƣ��ʼ��ԣ��ձ�����Һ����ɫΪ��ɫ��������ǡ����ȫ��Ӧʱ����Һ�����ԣ���Һ��ɫΪ��ɫ��������Ӧ�Ļ�ѧ����ʽΪNaOH+HCl==NaCl +H2O��
��2����pHͼ���֪�������Һ�����ԡ�����������ǹ����ġ�������Һ�в������������ơ����������Һ����ɫ����
��3����ͼ�Աȿ�֪�������Ӻ��������ڷ�Ӧ����Ȼ���ڡ����Է�Ӧ��ʵ����������Һ�е�������������������Һ�е����������ӽ�ϣ�������ˮ���������ᣨHClO3�����������أ�KOH����Ӧ�������κ�ˮ����ѧ����ʽΪ��KOH+ HClO3 ==KClO3 +H2O��
��4�����������֪����֪��Ϊ�������������δ֪��Ϊ����������Һ������������˼·���ɸ��ݴ����������������ڷ�Ӧ�е�������ϵ�����������������Һ������������������Ϊ��
�⣺��������������Һ������Ϊx��
NaOH + HCl==NaCl +H2O
40 36.5
20%x 36.5%��20g
40:36.5=20%x:36.5%��20g
x=40g

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
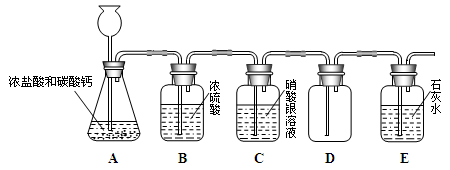
Сѧ��10����Ӧ����ϵ�д�����Ŀ����������������������Ҫ����֮һ��С��Ϊ̽��������������ˮ��Ӧ�����ᣬ���������ʵ�鷽����
��ʵ����ơ���������ɫʯ����Һ����ɫ��ֽ�������������ɫ��С����Ȼ������ͼʾ����ʵ�顣�۲쵽ʵ�飨I���ͣ�III���е�С����죬��II���е�С������ɫ��

�����۷�˼��С����С����ʵ�鷽����������ɣ�����Ϊ����ʵ�鲻����֤�������������ˮ��Ӧ��������������λͬѧ��ϸ���ۺ���Ϊ���貹������һ��ʵ�飬���ܵó�����������ˮ��Ӧ�����ᡣ
ʵ����� | ʵ������ |
__________________ | __________________ |
����չӦ�á���������Ҳ��ʵ����һ����Ⱦ���壬���ü�Һ���ա�д��ʵ����������������Һ���ն�������Ļ�ѧ����ʽ��_____________________________________________.