��Ŀ����
�����֣�ij��ѧС��ͬѧ������ͼ��ʾװ�ý���ʵ�顣

��1��A����̼���ƹ��壬B��������ʯ����Һ����ע����ע������ϡ���ᣬͬʱ��K��һ��ʱ��
�۲쵽B��������������˵�������������ԭ���ǣ��û�ѧ����ʽ��ʾ����
��2��A���Ŷ������̣�B����ľ̿��C�зų���ʯ��ˮ��Ϊ��
֤��ȼ��ȼ�յ���������������������ʵ�飺
�ٴˣ���ע����ע��һ���������������������������������
ľ̿��ȼ�գ��˲���Ŀ����������������
��һ��ʱ�����ע����ע�������Ĺ���������Һ��ͬʱ��K��A���������壬ľ̿��ȼ�գ�����ʯ��ˮδ����ǡ�������Ӧ�Ļ�ѧ����ʽΪ����������������
����������B����ȼ�ƾ��ƣ��۲쵽ľ̿ȼ�գ����⣬����ʯ��ˮ����ǣ��ô�������Ӧ�Ļ�ѧ������������ʽΪ ��

��1��A����̼���ƹ��壬B��������ʯ����Һ����ע����ע������ϡ���ᣬͬʱ��K��һ��ʱ��
�۲쵽B��������������˵�������������ԭ���ǣ��û�ѧ����ʽ��ʾ����
��2��A���Ŷ������̣�B����ľ̿��C�зų���ʯ��ˮ��Ϊ��
֤��ȼ��ȼ�յ���������������������ʵ�飺
�ٴˣ���ע����ע��һ���������������������������������
ľ̿��ȼ�գ��˲���Ŀ����������������
��һ��ʱ�����ע����ע�������Ĺ���������Һ��ͬʱ��K��A���������壬ľ̿��ȼ�գ�����ʯ��ˮδ����ǡ�������Ӧ�Ļ�ѧ����ʽΪ����������������
����������B����ȼ�ƾ��ƣ��۲쵽ľ̿ȼ�գ����⣬����ʯ��ˮ����ǣ��ô�������Ӧ�Ļ�ѧ������������ʽΪ ��
��1��ʯ����Һ��ɺ�ɫ CO2+H2O=H2CO3��2���ټ��ȣ´���֤���ﵽ�Ż�㵫������Ҳ����ȼ�ա� ���� 2H2O2 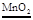 2H2O+O2���� ����CO2 + Ca(OH)2 ="=" CaCO3��+ H2O
2H2O+O2���� ����CO2 + Ca(OH)2 ="=" CaCO3��+ H2O
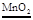 2H2O+O2���� ����CO2 + Ca(OH)2 ="=" CaCO3��+ H2O
2H2O+O2���� ����CO2 + Ca(OH)2 ="=" CaCO3��+ H2O���������1��A����̼���ƹ��壬B��������ʯ����Һ����ע����ע������ϡ���ᣬͬʱ��K��һ��ʱ��۲쵽B����������ʯ����Һ��ɺ�ɫ�������������ԭ����CO2+H2O=H2CO3��2���ٴˣ���ע����ע��һ�������������ȣ´���ľ̿��ȼ�գ��˲���Ŀ����֤���ﵽ�Ż�㵫������Ҳ����ȼ�ա�����һ��ʱ�����ע����ע�������Ĺ���������Һ��ͬʱ��K��A���������壬ľ̿��ȼ�գ�����ʯ��ˮδ����ǡ�������Ӧ�Ļ�ѧ����ʽΪ2H2O2
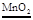 2H2O+O2��������B����ȼ�ƾ��ƣ��۲쵽ľ̿ȼ�գ����⣬����ʯ��ˮ����ǣ��ô�������Ӧ�Ļ�ѧ����ʽΪCO2 + Ca(OH)2 ="=" CaCO3��+ H2O
2H2O+O2��������B����ȼ�ƾ��ƣ��۲쵽ľ̿ȼ�գ����⣬����ʯ��ˮ����ǣ��ô�������Ӧ�Ļ�ѧ����ʽΪCO2 + Ca(OH)2 ="=" CaCO3��+ H2O
��ϰ��ϵ�д�
 �����Ƹ���ʦ����ϵ�д�
�����Ƹ���ʦ����ϵ�д� ��ͨ����ͬ����ϰ��ϵ�д�
��ͨ����ͬ����ϰ��ϵ�д�
�����Ŀ
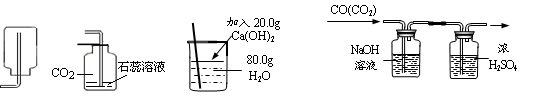

 W+3H2O�еĻ�ԭ����
W+3H2O�еĻ�ԭ����