��Ŀ����
����Ŀ��ij��ȤС����Ҫ����100g��������Ϊ9.8%��ϡ���ᣬ���賣���¸�ϡ�����pHֵΪ0����װ��100g��ϡ������ձ��м���104.4gNa2CO3��Һ��ǡ����ȫ��Ӧ����Һ�����ԣ�
��1��ʵ����ֻ��98%��Ũ���ᣬ�ܶ�Ϊ1.84g/cm3 �� ��Ҫ��ȡmL������һλС����Ũ�������ϡ�ͣ�ϡ��Ũ����ķ����� ��
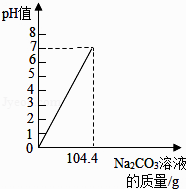
��2������ͼ�У������ձ�����Һ��pHֵ�仯ͼ�����Na2CO3��Һ�������� 
��3������������Һ���������������Ƕ��٣���д��������̣�
���𰸡�
��1��5.4����Ũ�������ձ��ڻ���ע��ˮ�У������ò��������Ͻ���
��2���⣺����104.4gNa2CO3��Һ��ǡ����ȫ��Ӧ����ʱ��Һ��pH=7������̼������Һ�ļ��룬pH���ߣ�����ͼ��ʾ��
��3���⣺�����������Ƶ�����Ϊy�����ɶ�����̼������Ϊz��
Na2CO3+ | H2SO4 | �T | Na2SO4+ | H2O+ | CO2�� |
98 | 142 | 44 | |||
100g��9.8% | y | z |
![]() =
= ![]() =
= ![]() ��
��
y=14.2g��z=4.4g��
������Һ��������������Ϊ�� ![]() ��100%=7.1%��
��100%=7.1%��
��������Һ��������������Ϊ7.1%
���������⣺��1������ҪŨ��������Ϊx�� ���У�x��1.84g/cm3��98%=100g��9.8%��
x=5.4mL��
ϡ��Ũ����ķ����ǣ���Ũ�������ձ��ڻ���ע��ˮ�У������ò��������Ͻ��裮
���5.4����Ũ�������ձ��ڻ���ע��ˮ�У������ò��������Ͻ��裮
�����㾫����������Ĺؼ�����������ݻ�ѧ��Ӧ����ʽ�ļ�������֪ʶ�����ո����ʼ�������=ϵ������Է�������֮�ȣ�