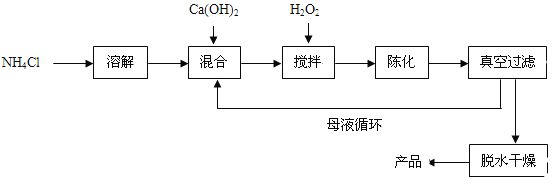
��Ŀ����
����Ŀ�� ��ѧʵ����ϣ���ʦΪÿ��ͬѧ�ֱ��ṩ��һƿ����������Һ����������1%��ϡ���ᣨ�ܶ�Ϊ1.1g/mL�����ⶨ�����ʵ���������������ͬѧ��ʵ����ͼ1��ʾ�����ձ��м���5g����������Һ�����뼸�η�̪��Һ���õι���������1%��ϡ���ᣬ�����Ͻ���, ��ҺpH�ı仯��ͼ2��ʾ��
( ��Ӧ�Ļ�ѧ����ʽΪNaOH + HCl === NaCl + H2O )
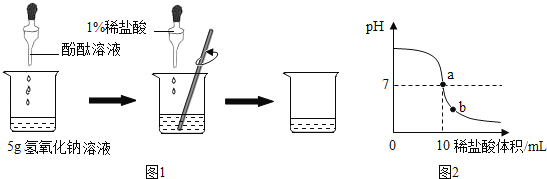
��ش𣺣�1����ͼ2��ʾ��a����Һ�д��ڵ���������__________�����ӷ��ű�ʾ��
��2��ǡ����ȫ��Ӧʱ������ϡ�������������__________ g��
��3����������������Һ�����ʵ���������������ȷ��0.1%��
���𰸡���1�� Na+ ��2�� 0.11 ��3��2.4%
��������
�����������1��a���ʾ���ǡ����ȫ��Ӧ����NaCl ��H2O������Һ�е�������ΪNa+
(2) ǡ����ȫ��Ӧʱ������ϡ��������Ϊ10ml������������=10ml��1.1g/ml��1%=0.11g
��3���⣺������������Һ�����ʵ�����Ϊx��
NaOH + HCl == NaCl + H2O
40 36.5
x 0.11g
![]()
![]() =
=![]() X=0.12 g
X=0.12 g
NaOH%=![]() ��100% =2.4��
��100% =2.4��
������������Һ�����ʵ���������Ϊ2.4��.

��ϰ��ϵ�д�
�����Ŀ