��Ŀ����
������������ˮ�����ӻ�����A��B��C��D�������ɱ��е������γɵģ����κ����������ж���������ͬ�����ӣ�Ϊ�������ǣ��ֱ��������ʵ�飨����ÿ�η�Ӧ��ǡ����ȫ���������ǣ�
��A��B����Һ������ɰ�ɫ������C��D����Һ������ɰ�ɫ������
��ʵ�����ʵ��ڵ���Һ��Ϻ���Ȳ���ʹ��ɫʯ����ֽ���������壻
��C��Һ�ʼ��ԣ������м������ϡ���ᣬ������ɫ���壻
����A��Һ�м���ϡ���������ɫ������
д��A B C D�Ļ�ѧʽA _________ ��B _________ ��C _________ ��D _________
��ʵ�����ʵ��ڵ���Һ��Ϻ���Ȳ���ʹ��ɫʯ����ֽ���������壻
��C��Һ�ʼ��ԣ������м������ϡ���ᣬ������ɫ���壻
����A��Һ�м���ϡ���������ɫ������
д��A B C D�Ļ�ѧʽA _________ ��B _________ ��C _________ ��D _________
A. AgNO3 ��B. NH4Cl ��C. Na2CO3 �� D . Ba(OH)2

��ϰ��ϵ�д�
�����Ŀ
������������ˮ�����ӻ�����A��B��C��D�������ɱ��е������γɵģ����κ����������ж���������ͬ�����ӣ�
Ϊ�������ǣ��ֱ��������ʵ�飨����ÿ�η�Ӧ��ǡ����ȫ���������ǣ�
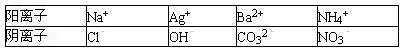
| ������ | Na+ | Ag+ | Ba2+ | NH4+ |
| ������ | Cl- | OH- | CO32- | NO3- |
��ʵ�����ʵ��ڵ���Һ��Ϻ���Ȳ���ʹ��ɫʯ����ֽ���������壻��C��Һ�ʼ��ԣ������м������ϡ���ᣬ������ɫ���壻
����A��Һ�м���ϡ���������ɫ������д��A B C D�Ļ�ѧʽ
A________��B________��C________��D________��
������������ˮ�����ӻ�����A��B��C��D�������ɱ��е������γɵģ����κ����������ж���������ͬ�����ӣ�
Ϊ�������ǣ��ֱ��������ʵ�飨����ÿ�η�Ӧ��ǡ����ȫ���������ǣ�
��A��B����Һ������ɰ�ɫ������C��D����Һ������ɰ�ɫ������
��ʵ�����ʵ��ڵ���Һ��Ϻ���Ȳ���ʹ��ɫʯ����ֽ���������壻��C��Һ�ʼ��ԣ������м������ϡ���ᣬ������ɫ���壻
����A��Һ�м���ϡ���������ɫ������д��A B C D�Ļ�ѧʽ
A______��B______��C______��D______��
Ϊ�������ǣ��ֱ��������ʵ�飨����ÿ�η�Ӧ��ǡ����ȫ���������ǣ�
| ������ | Na+ | Ag+ | Ba2+ | NH4+ |
| ������ | Cl- | OH- | CO32- | NO3- |
��ʵ�����ʵ��ڵ���Һ��Ϻ���Ȳ���ʹ��ɫʯ����ֽ���������壻��C��Һ�ʼ��ԣ������м������ϡ���ᣬ������ɫ���壻
����A��Һ�м���ϡ���������ɫ������д��A B C D�Ļ�ѧʽ
A______��B______��C______��D______��