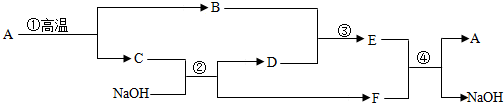
��Ŀ����
A��B��C��D��E��F�dz��л�ѧ�г��������ʣ��������ǵ�ת����ϵ�ش��������⣺

��1��д���������ʵ����ƣ�����E��
��2�����鼯��ƿ������IJ���������
��3���ڷ�Ӧ���У�����B��
��4��д�����л�ѧ��Ӧ���ֱ���ʽ��������Ӧ���ͣ�
��Ӧ�٣�
��Ӧ�ڣ�
��Ӧ�ܣ�

��1��д���������ʵ����ƣ�����E��
����������
����������
������������
����
����2�����鼯��ƿ������IJ���������
�������ǵ�ľ���쵽����ƿ�ڣ��۲�ľ���Ƿ�ȼ
�������ǵ�ľ���쵽����ƿ�ڣ��۲�ľ���Ƿ�ȼ
����3���ڷ�Ӧ���У�����B��
��
��
���ã���4��д�����л�ѧ��Ӧ���ֱ���ʽ��������Ӧ���ͣ�
��Ӧ�٣�
2KMnO4
K2MnO4+MnO4+O2��
| ||
2KMnO4
K2MnO4+MnO4+O2��
��
| ||
�ֽ�
�ֽ�
��Ӧ����Ӧ�ڣ�
4P+5O2
2P2O5
| ||
4P+5O2
2P2O5
��
| ||
����
����
��Ӧ����Ӧ�ܣ�
2H2O
2H2��+O2��
| ||
2H2O
2H2��+O2��
��
| ||
�ֽ�
�ֽ�
��Ӧ�����������ݿ�ͼ��Ϣ��A���Ϻ�ɫ���壬��A�Ǹ�����أ����ȷֽ����ɵ����������������ɫ����B����ҺD��ϲ�������ɫҺ��ͨ���ֽܷ�������������B�Ƕ������̣�D�ǹ������⣬���ɵ��������������������ɵĹ���F������أ�����C��������ȼ�ղ�������E����C�����Ǻ��ף�E�����������ף��ݴ˽��
����⣺A���Ϻ�ɫ���壬��A�Ǹ�����أ����ȷֽ����ɵ����������������ɫ����B����ҺD��ϲ�������ɫҺ��ͨ���ֽܷ�������������B�Ƕ������̣�D�ǹ������⣬���ɵ��������������������ɵĹ���F������أ�����C��������ȼ�ղ�������E����C�����Ǻ��ף�E�����������ף������ͼ���ƶϺ�����
��1������E�����������ף���������������������������ף�������
��2���������������������ǽ������ǵ�ľ���쵽����ƿ�ڣ��۲�ľ���Ƿ�ȼ������������ǵ�ľ���쵽����ƿ�ڣ��۲�ľ���Ƿ�ȼ��
��3��B�Ƕ������̣��ڹ�������ֽ���������ã��������
��4����Ӧ�٣�A�Ǹ�����أ����ȷֽ�����������ء��������̺����������ڷֽⷴӦ�����2KMnO4
K2MnO4+MnO4+O2�����ֽ⣻
��Ӧ�ڣ�����������C�Ǻ��ף�������������ȼ���������������ף����ڻ��Ϸ�Ӧ�����4P+5O2
2P2O5�����ϣ�
��Ӧ�ܣ�ˮͨ�����������������������ڷֽⷴӦ�����2H2O
2H2��+O2�����ֽ⣮
��1������E�����������ף���������������������������ף�������
��2���������������������ǽ������ǵ�ľ���쵽����ƿ�ڣ��۲�ľ���Ƿ�ȼ������������ǵ�ľ���쵽����ƿ�ڣ��۲�ľ���Ƿ�ȼ��
��3��B�Ƕ������̣��ڹ�������ֽ���������ã��������
��4����Ӧ�٣�A�Ǹ�����أ����ȷֽ�����������ء��������̺����������ڷֽⷴӦ�����2KMnO4
| ||
��Ӧ�ڣ�����������C�Ǻ��ף�������������ȼ���������������ף����ڻ��Ϸ�Ӧ�����4P+5O2
| ||
��Ӧ�ܣ�ˮͨ�����������������������ڷֽⷴӦ�����2H2O
| ||
���������⿼�������ʵ��ƶϣ���ɴ��⣬�������ݿ�ͼ��Ϣ������е�֪ʶ���У���ɴ��⣬��Ҫͬѧ���ܸ�����������������ͻ�ƿڣ�ֱ�ӵó��й����ʵĻ�ѧʽ��Ȼ���ƶϵó��������ʵĻ�ѧʽ��

��ϰ��ϵ�д�
 Ӧ�����������Ĵ���ѧ������ϵ�д�
Ӧ�����������Ĵ���ѧ������ϵ�д�
�����Ŀ
 ��2010?����һģ�����ʵ��ƶϣ�
��2010?����һģ�����ʵ��ƶϣ� ��2004?¬������ģ��A��B��C��D��E��F��G�������ʣ�A��C����ɫ���壨�������B��D���Ǻ�ɫ��ĩ��
��2004?¬������ģ��A��B��C��D��E��F��G�������ʣ�A��C����ɫ���壨�������B��D���Ǻ�ɫ��ĩ�� ��֪��A ����Է���������С�������D ������л��Ҳ����Ȼ��������Ҫ�ijɷ֣������A��B��C��D��E ��������֮��ı仯��ϵ���ش��й����⣮
��֪��A ����Է���������С�������D ������л��Ҳ����Ȼ��������Ҫ�ijɷ֣������A��B��C��D��E ��������֮��ı仯��ϵ���ش��й����⣮