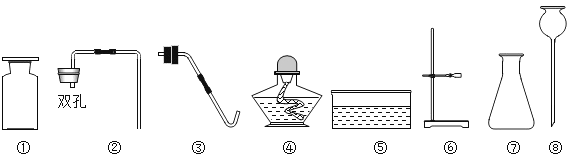
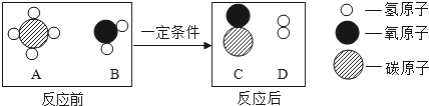
��Ŀ����
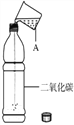
����Ŀ������ͼ��ʾ������֧����������̼���Թֱܷ�����ʢ��ˮ������ʯ��ˮ��Ũ����������Һ�н���ʵ�飬�밴Ҫ��ش�������⣺
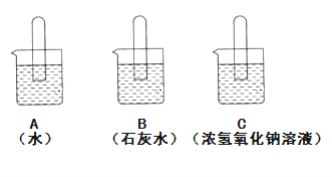
����ʾ��ͼʾ�г��ֵ���ʵ������е��Թ���Һ��ĸ߶ȣ��������յ�����
(1)B�е�ʵ��������________����Ӧ�Ļ�ѧ����ʽΪ________��
(2)֤��A�ж�����̼����ˮ��Ӧ�ķ�����________��
(3)�Ƿ����ͨ������ʵ���е���������֤��������̼�����������Ʒ�Ӧ����������________��
���𰸡�����ʯ��ˮ����ǣ��Թ���Һ��������Ca(OH)2+CO2=CaCO3��+H2O����Ĵָ��ס�Թܿ��Ƴ�ˮ������������Թ��ڵ���2-3����ɫʯ����Һ����Һ���ֺ�ɫ������ͨ���Ա�ʵ��A��C֤������������C��A�Աȣ������е�����ˮ֮�⣬�ֺ����������ƣ���C�н����Թ���Һ���Һ���ָ���A�е�Һ�棬�ʿ���֤��������̼���������Ʒ�����Ӧ
��������
�����������֪��
(1)B�е�ʵ�������dz���ʯ��ˮ����ǣ��Թ���Һ����������Ӧ�Ļ�ѧ����ʽΪCa(OH)2+CO2=CaCO3��+H2O��
(2))֤��A�ж�����̼����ˮ��Ӧ�ķ�������Ĵָ��ס�Թܿ��Ƴ�ˮ������������Թ��ڵ���2-3����ɫʯ����Һ����Һ���ֺ�ɫ��
(3)����ͨ���Ա�ʵ��A��C֤������������C��A�Աȣ������е�����ˮ֮�⣬�ֺ����������ƣ���C�н����Թ���Һ���Һ���ָ���A�е�Һ�棬�ʿ���֤��������̼���������Ʒ�����Ӧ��