��Ŀ����
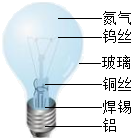 ��2013?���룩û�н�������������������ģ�������ݶԽ����ͽ������ϵ���ʶ���ش��������⣮
��2013?���룩û�н�������������������ģ�������ݶԽ����ͽ������ϵ���ʶ���ش��������⣮��1����ͼ���������ɵ��ݵ����ʣ�������������в����ڽ������ϵ���
����������
����������
����2�������������ó�����ʯұ��������������ʯ����Ҫ�ɷ���
Fe2O3
Fe2O3
���ܶ���ͳ��ݵĽ���ʹ���˴����ĸֲĶ����Ǵ�������Ҫ����Ϊ�ֱȴ�������Ӳ�ȴ�
Ӳ�ȴ�
���������ܣ���3������ʦ���и�����ʱ��������ͭ��Һ�������ϻ��߿������º�ɫ�ۼ����ñ仯��ԭ����
Fe+CuSO4=FeSO4+Cu
Fe+CuSO4=FeSO4+Cu
���û�ѧ����ʽ��ʾ������������1�����ݽ������ϵ��ص�������
��2�����ݳ�����ijɷ��Լ��������������������������ɣ�
��3�����ݽ������˳���Ӧ�÷������
��2�����ݳ�����ijɷ��Լ��������������������������ɣ�
��3�����ݽ������˳���Ӧ�÷������
����⣺��1������������Ҫ�ǰ����������ʺͽ����Ͻ�����������������в����ڽ������ϵ��Dz����͵�����
��2�����������Ҫ�ɷ������������������������ڴ��ͳ��ݵĽ���ʹ�õ���Ӳ�Ƚϴ�ĸֶ����Ǵ�����
��3���������Ľ�����Ա�ͭǿ���������ܹ��û�������ͭ�е�ͭ����������ͭ��Һ�������ϻ��߿������º�ɫ�ۼ����ñ仯��ԭ����Fe+CuSO4=FeSO4+Cu
�ʴ�Ϊ����1����������������2��Fe2O3�������������������������� Ӳ�ȴ�Ӳ�ȴ����ʴ������3��Fe+CuSO4=FeSO4+Cu��
��2�����������Ҫ�ɷ������������������������ڴ��ͳ��ݵĽ���ʹ�õ���Ӳ�Ƚϴ�ĸֶ����Ǵ�����
��3���������Ľ�����Ա�ͭǿ���������ܹ��û�������ͭ�е�ͭ����������ͭ��Һ�������ϻ��߿������º�ɫ�ۼ����ñ仯��ԭ����Fe+CuSO4=FeSO4+Cu
�ʴ�Ϊ����1����������������2��Fe2O3�������������������������� Ӳ�ȴ�Ӳ�ȴ����ʴ������3��Fe+CuSO4=FeSO4+Cu��
�����������Ҫ���ջ�ѧ����ʽ����д�����ͽ��������ʵȷ����֪ʶ��ֻ���������ܶ���ط��������������ȷ���жϣ�

��ϰ��ϵ�д�
�����Ŀ