��Ŀ����
��2009?�人��Ϊ�ⶨʯ��ʯ��Ʒ��̼��Ƶ�����������̽��С���ͬѧ�ֱ���ͬ��������Ʒ�м���100gϡ���ᣬ�õ��������±���֪����Ʒ�к��е�����������ˮ���Ҳ������ᷴӦ��
�����Ҫ��ش���������
��1����������̼����ͨ�������ɫʯ����Һ������ˮ�У���ʯ����Һ��
��2�������ʯ��ʯ��Ʒ��̼��Ƶ�����������
| ʵ����� | 1 | 2 | 3 |
| ϡ�����������g�� | 100 | 100 | 100 |
| ʯ��ʯ��������g�� | 8 | 18 | 28 |
| ������̼��������g�� | 2.2 | 4.4 | 4.4 |
��1����������̼����ͨ�������ɫʯ����Һ������ˮ�У���ʯ����Һ��
��
��
ɫ����2�������ʯ��ʯ��Ʒ��̼��Ƶ�����������
��������1��������ɫʯ����Һ��������Һ���ɫ�������Һ����ɫ��������
��2������ʯ��ʯ��Ʒ��̼�����ȫ��Ӧ���Ǵβ���������̼�����������̼��Ƶ���������̼��Ƶ���������ʯ��ʯ��Ʒ���������ɣ�
��2������ʯ��ʯ��Ʒ��̼�����ȫ��Ӧ���Ǵβ���������̼�����������̼��Ƶ���������̼��Ƶ���������ʯ��ʯ��Ʒ���������ɣ�
����⣺��1��������̼����ͨ�������ɫʯ����Һ������ˮ�У�������̼��ˮ��Ӧ����̼�ᣬ̼����Һ��������ʹ��ɫʯ����Һ���ɫ��
�ʴ�Ϊ����
��2����ͼ��֪100gϡ�����м���8g��Ʒ����2.2g ������̼��100gϡ�����м���18g��Ʒ����4.4g ������̼��˵����ʵ��1��������ʣ�̼࣬�����ȫ��Ӧ���ʿɸ���ʵ��1�ж�����̼����������̼��Ƶ�������
��8gʯ��ʯ��Ʒ��̼��Ƶ�����Ϊx
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
x 2.2g
=
x=5g
ʯ��ʯ��̼��Ƶ���������
��100%=62.5%
��ʯ��ʯ��̼��Ƶ���������62.5%��
�ʴ�Ϊ����
��2����ͼ��֪100gϡ�����м���8g��Ʒ����2.2g ������̼��100gϡ�����м���18g��Ʒ����4.4g ������̼��˵����ʵ��1��������ʣ�̼࣬�����ȫ��Ӧ���ʿɸ���ʵ��1�ж�����̼����������̼��Ƶ�������
��8gʯ��ʯ��Ʒ��̼��Ƶ�����Ϊx
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
x 2.2g
| 100 |
| x |
| 44 |
| 2.2g |
x=5g
ʯ��ʯ��̼��Ƶ���������
| 5g |
| 8g |
��ʯ��ʯ��̼��Ƶ���������62.5%��
������ͨ���ش���֪����ͨ��ͼ�����ݷ����Ĵ���ȫ��Ӧ���Ĵ���ʣ��ķ���������һ�������˼�����Ľ��ⲽ�裬�����Ĺؼ��Ƿ�������һ��̼����Ѿ�����Ӧ���ˣ��ٽ�һ��������֤���ɣ�

��ϰ��ϵ�д�
�����Ŀ
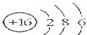 ��2009?�人����ͼΪijԪ�ص�ԭ���ܽṹʾ��ͼ�������й�˵�������ǣ�������
��2009?�人����ͼΪijԪ�ص�ԭ���ܽṹʾ��ͼ�������й�˵�������ǣ�������