��Ŀ����
(5��)ij��ѧ��ȤС�����ⶨijʯ��ʯ��Ʒ��̼��Ƶ�����������ȡ20gʯ��ʯ��Ʒ(�������ʼȲ�����ˮ��Ҳ�����������ʷ�Ӧ)�������м���100g������������Ϊ10��95����ϡ���ᣬǡ����ȫ��Ӧ����ش��������⡣
(1)������100g����ʵ�����õ�ϡ���ᣬ��Ҫ36��5����Ũ��������Ϊ________________
(2)д����Ӧ�Ļ�ѧ����ʽ ________________
(3)�г�������Ʒ��μӷ�Ӧ��̼�������(x)�ı���ʽ ________
(4) ����Ʒ��̼��Ƶ���������Ϊ________
(5)��Ӧ��Ĺ�Һ������м���l13��6gˮ����ֽ������ˣ��õ�ֻ��һ�����ʵIJ�������Һ�������ò�������Һ�����ʵ���������Ϊ________________
��1��30g
��2��CaCO+2HCl=CaCl2+H2O+CO2����3��100/73="X/10.95g" (4)7.5%
����

 �Ķ��쳵ϵ�д�
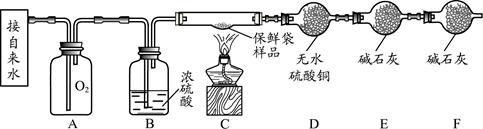
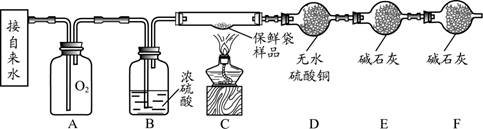
�Ķ��쳵ϵ�д�������ͼ�ش����⡣
 |
��1������a�������� ��
��2��ʵ������ȡ������̼��ѡ�õķ���װ���� ����װ����ţ���ͬ������ѡ��װ��E�ռ�������̼��ԭ����______________________��
��3��ʵ�����ø��������ȡ������Ӧ�Ļ�ѧ����ʽ�� _____________________ ��
��ѡ�õ��ռ�װ����E�� ����Ҫ��ʵ�鲽���У��ټ��� ��װҩƷ���̶�
�Թ� �ۼ��װ�õ������� ������ˮ���ռ����� ��ֹͣ���� �����ܴ�ˮ
����ȡ������ȷ�IJ���˳���� _____________ ������ű�ʾ����
��1���ƾ��ƣ�2��B ������̼������ˮ����ˮ��Ӧ��ֻ��һ����֣�
|
32����5�֣�32����5�֣�ij��ѧС��ⶨ���������������������
 ��1��������ͼ�ش����⡣
��1��������ͼ�ش����⡣
�ٺ���ȼ�յĻ�ѧ����ʽΪ__________________________________��
����������Ϊľ̿����Ӧ�����������£���ֹˮ�У��ձ�
�е�ˮ�������뼯��ƿ��ԭ����______________________________
 _______________________________________________��
_______________________________________________��
��2����������(Na2S4)�����������ײⶨ���������������������
��Ӧԭ��Ϊ:2Na2S4+O2+2H2O 8S��+4NaOH���������ƣ���
С���ϣ�������(Na2S4)��������ˮ��Ӧ����������ˮ�Ĺ�����S����������ˮ
���������ơ�
��ʵ����̡�
��ȡ�����������ƹ�������Թ��У��ټ���������ˮ��Ѹ���������������������Һ�����������صľ��룬��¼����h1(��ͼ1��ʾ)��
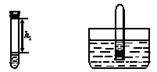
ͼ1 ͼ2
�ڽ����Թܲ���ˮ��(��ͼ2��ʾ)�������������۲쵽__________���������������Թ�ȡ������ת����������Һ�����������صľ��룬��¼����h2��������h2��h1= ��
�۰��բٺ͢� ���ظ�ʵ��2�Ρ�3��ʵ���������±���ʾ��
���ظ�ʵ��2�Ρ�3��ʵ���������±���ʾ��
| ��1�� | ��2�� | ��3�� | |
| h1/cm | 11.0 | 11.4 | 11.6 |
| h2/cm | 8.7 | 9.1 | 9.2 |
���ݵ�3��ʵ�����ݣ�����������������������Ϊ %(�����ȷ�� 0.1%)��
��1����4P + 5O 2 2P2O5
2P2O5
�� ��Ϊľ̿ȼ�����ɶ�����̼���壬����ƿ�������������,ƿ������ѹǿû�����Ա�
��Ϊľ̿ȼ�����ɶ�����̼���壬����ƿ�������������,ƿ������ѹǿû�����Ա�
�������ԣ�����ֹˮ��ʱˮ�������뵽����ƿ�С�
��2�����Թ���Һ��������Һ��߶Ȳ��ٸı� 79��100(��4��5)  �� 20.7
�� 20.7
33��(8��)ij��ȤС��ͬѧ�Ķ��������ϵ�֪���ܶ����ض���Ӱ���������ֽ��ٶȡ����ǣ�
ͬѧ��̽��Ӱ���������ֽ��ٶȵ�ij�����ء�
��ʵ����̡�ʵ�����ݼ�¼���£�
| ����������Һ������ | ����������Һ��Ũ�� | MnO2������ | �������� | |
| �� | 50.0g | 1% | 0.1g | 9 mL |
| �� | 50.0g | 2% | 0.1g | 16 mL |
| �� | 50.0g | 4% | 0.1g | 31 mL |
��ʵ�������
��1����������ֽ�Ļ�ѧ����ʽΪ______________________________________��
��2�������еġ��������ݡ���ָ���ǣ���ͬʱ����_________________________________��
��3����ʵ���У�����O2�����װ����________�����ţ���
 |
��ʵ����ۡ�
����ͬ������______________________________________________��
���������ۡ�
��1����ͬѧ���������ʵ���в���______________ͬ���ܵó���ͬ�Ľ��ۡ�
 ��2����ͬѧ�������ͼװ�ý���ʵ�飬ͨ���Ƚ�_____________
��2����ͬѧ�������ͼװ�ý���ʵ�飬ͨ���Ƚ�_____________
___________________Ҳ�ܴﵽʵ��Ŀ�ġ�
��ʵ�鷴˼��
�����������ػ�Ӱ���������ֽ��ٶ��⣬ ___________��д
һ�֣�Ҳ��Ӱ���������ֽ��ٶȣ����ʵ�鷽����______________________________________________________________________��