��Ŀ����
̼��̼�Ļ������ڹ�ũҵ�������ճ��������й㷺����;��
��ͼ��̼ԭ�ӽṹʾ��ͼ��̼ԭ�ӵ���������________��̼Ԫ����Ԫ�����ڱ���λ�ڵ�______���ڡ�
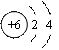
��2���Ŵ���ī��д����Ƶ��ֻ�������Բ���ɫ��ԭ����ī����Ҫ�ɷ�̼�ڳ����¾���______�ԡ�
��3��������̼�ܲ�����������ɴ����еġ�̼ѭ���������Ǵ����еĶ�����̼�ĺ���������������ʹȫ���ů���Ӷ�����_____________���߲ˡ��������������ʡ��Ķ�����̼�����ö��ַ����Ƶã����������ϡ���ᣨH2SO4����̼����泥�NH4HCO3����Ӧ�Ƶã���Ӧ����������泥�ˮ�Ͷ�����̼���÷�Ӧ�Ļ�ѧ����ʽΪ______________________
��ͼ��̼ԭ�ӽṹʾ��ͼ��̼ԭ�ӵ���������________��̼Ԫ����Ԫ�����ڱ���λ�ڵ�______���ڡ�
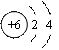
��2���Ŵ���ī��д����Ƶ��ֻ�������Բ���ɫ��ԭ����ī����Ҫ�ɷ�̼�ڳ����¾���______�ԡ�
��3��������̼�ܲ�����������ɴ����еġ�̼ѭ���������Ǵ����еĶ�����̼�ĺ���������������ʹȫ���ů���Ӷ�����_____________���߲ˡ��������������ʡ��Ķ�����̼�����ö��ַ����Ƶã����������ϡ���ᣨH2SO4����̼����泥�NH4HCO3����Ӧ�Ƶã���Ӧ����������泥�ˮ�Ͷ�����̼���÷�Ӧ�Ļ�ѧ����ʽΪ______________________
6�������ȶ�������ЧӦ��H2SO4+2NH4HCO3=(NH4)2SO4+2H2O+2CO2��
����1���⣺��̼ԭ�ӽṹʾ��ͼ����֪��̼ԭ����������Ϊ6������Ԫ�������ɣ����Ӳ�������������������̼Ԫ��λ�ڵڶ����ڣ�
��2��̼��ͨ������»�ѧ���ʲ����ã�һ�㲻�����������ʷ�����ѧ��Ӧ��������̼�ڳ����µ��ȶ��ԣ��ʹŴ��ֻ�����Բ���ɫ��
��3��������̼�������γ�����ЧӦ����Ҫ���壬������̼�����������������������֮Ϊ����ЧӦ��̼����������ᷢ�����ֽⷴӦ��ͨ�������ɷֶ���������李�ˮ�Ͷ�����̼���ʴ�Ϊ����ЧӦ��2NH4HCO3+H2SO4�T��NH4��2SO4+2H2O+2CO2����
�ʴ�Ϊ����1��6 ����
��2���ȶ���
��3������ЧӦ��2NH4HCO3+H2SO4�T��NH4��2SO4+2H2O+2CO2����
��2��̼��ͨ������»�ѧ���ʲ����ã�һ�㲻�����������ʷ�����ѧ��Ӧ��������̼�ڳ����µ��ȶ��ԣ��ʹŴ��ֻ�����Բ���ɫ��
��3��������̼�������γ�����ЧӦ����Ҫ���壬������̼�����������������������֮Ϊ����ЧӦ��̼����������ᷢ�����ֽⷴӦ��ͨ�������ɷֶ���������李�ˮ�Ͷ�����̼���ʴ�Ϊ����ЧӦ��2NH4HCO3+H2SO4�T��NH4��2SO4+2H2O+2CO2����
�ʴ�Ϊ����1��6 ����
��2���ȶ���
��3������ЧӦ��2NH4HCO3+H2SO4�T��NH4��2SO4+2H2O+2CO2����

��ϰ��ϵ�д�
�����Ŀ