��Ŀ����
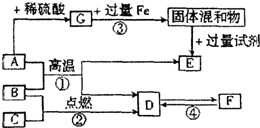 ��ͼA��G�dz��л�ѧ���������ʣ�����AΪ��ɫ���壬EΪ��ɫ���壬B��C��Dͨ����Ϊ��ɫ���壬FΪһ�ֲ�����Ԫ�ص����Σ�G��ҺΪ��ɫ���ת����ϵ��ͼ���������ͼ�ش��������⣺
��ͼA��G�dz��л�ѧ���������ʣ�����AΪ��ɫ���壬EΪ��ɫ���壬B��C��Dͨ����Ϊ��ɫ���壬FΪһ�ֲ�����Ԫ�ص����Σ�G��ҺΪ��ɫ���ת����ϵ��ͼ���������ͼ�ش��������⣺��1������A��F�Ļ�ѧʽ�ֱ�Ϊ
CuO
CuO
��Na2CO3
Na2CO3
����2����Ӧ�۵Ļ�ѧ����ʽΪ
Fe+CuSO4�TFeSO4+Cu
Fe+CuSO4�TFeSO4+Cu
����3�����л�ù�������IJ���������
����
����
���������������������
����
���ù����������������ʵĻ�ѧʽ��Fe��Cu
Fe��Cu
��Ҫ���з����E���ʣ����ӵĹ����Լ���������ϡ���ᣨ��ϡ���ᣩ��ͭ���ӵ�����Һ
ϡ���ᣨ��ϡ���ᣩ��ͭ���ӵ�����Һ
����4������ת��������û���漰�Ļ�ѧ��Ӧ����������
�ֽ�
�ֽ�
��Ӧ��������GΪ��ɫ��Һ����ͭ������Һ��A�����ᷴӦ����G������G������ͭ��AΪ��ͭ�ĺ�ɫ���壬����������ͭ��E������ͭ����ԭ�Ƶã������ں�ɫ���嵥�ʣ�����E��ͭ��FΪ��������Ԫ�ص����Σ�����D���ת����D��������ͭ����ԭ��IJ���ٸ�������ԭ�����п��ǣ�D�����������Ƶ�̼���ƣ�����D�Ƕ�����̼��A��B��Ӧ����ͭ�Ͷ�����̼��B��C������̼��˵��C����ȼ�Ե��������ٸ��ݷ���ʽ��д�������ʵķ��뷽���ش��⣮
����⣺��1��ͭ������Һ����ɫ��GΪ��ɫ��Һ��������ͭ������Һ��A�����ᷴӦ����G�������û���Ӧ��������G������ͭ��AΪ��ͭ�ĺ�ɫ���壬����������ͭ��E������ͭ����ԭ���Ƶã������ں�ɫ���嵥�ʣ�����E��ͭ��FΪ��������Ԫ�ص����Σ�D��F���ת����D�ǻ�ԭ����������ȼ�Ե��������ɵ�ˮ�������̼�����ڻ��Ϸ�Ӧ����ֻ�ж�����̼����̼�����ת��������D�Ƕ�����̼��F��̼���ƣ�����̼���ƺ��ᷴӦ���ڸ��ֽⷴӦ����
��2����ΪG������ͭ���ʿ��Ժ��������û���Ӧ��Fe+CuSO4�TFeSO4+Cu��
��3���������Һ�������ù��˷������в��������������ã����������������ɵ�ͭ����������Ҫ��ͭ������������Լ�����������Ӧ�����ǿ�����û��������ķ������ʴ�Ϊ��ϡ���ᣨ��ϡ���ᣩ��ͭ���ӵ�����Һ��
��4�������ķ�Ӧ�������������û���Ӧ�����ֽⷴӦ�����Ϸ�Ӧ��û�зֽⷴӦ��
�ʴ�Ϊ����1��CuO Na2CO3 ��2��Fe+CuSO4�TFeSO4+Cu ��3������ ���� Fe��Cu ϡ���ᣨ��ϡ���ᣩ��ͭ���ӵ�����Һ ��4���ֽ⣮
��2����ΪG������ͭ���ʿ��Ժ��������û���Ӧ��Fe+CuSO4�TFeSO4+Cu��
��3���������Һ�������ù��˷������в��������������ã����������������ɵ�ͭ����������Ҫ��ͭ������������Լ�����������Ӧ�����ǿ�����û��������ķ������ʴ�Ϊ��ϡ���ᣨ��ϡ���ᣩ��ͭ���ӵ�����Һ��
��4�������ķ�Ӧ�������������û���Ӧ�����ֽⷴӦ�����Ϸ�Ӧ��û�зֽⷴӦ��
�ʴ�Ϊ����1��CuO Na2CO3 ��2��Fe+CuSO4�TFeSO4+Cu ��3������ ���� Fe��Cu ϡ���ᣨ��ϡ���ᣩ��ͭ���ӵ�����Һ ��4���ֽ⣮
�����������ؼ����ҳ�ͻ�ƿڣ�GΪ��ɫ��Һ��ͭ������Һ��AΪ��ɫ���������ᷴӦ����G������G������ͭ������Ҫ֪����ɫ�����У���������ͭ���������̡�������������̼�����ʣ�AΪ��ͭ�ĺ�ɫ���壬����������ͭ���ٽ�һ�����з������ɣ��������ṩ�Ŀ��ܴ��ڵ����ʵ����ʣ����ʵ��������ȷ�����ʣ��ǽ������ʵ���ƶ���������������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ



 ��ͼA��G�dz��л�ѧ���������ʣ�����AΪ��ɫ���壬EΪ��ɫ���嵥�ʣ�B��C��D��Ϊ��ɫ���壬FΪʯ��ʯ����Ҫ�ɷ֣�GΪ��ɫ��Һ�������ͼ�ش��������⣺
��ͼA��G�dz��л�ѧ���������ʣ�����AΪ��ɫ���壬EΪ��ɫ���嵥�ʣ�B��C��D��Ϊ��ɫ���壬FΪʯ��ʯ����Ҫ�ɷ֣�GΪ��ɫ��Һ�������ͼ�ش��������⣺ ��ͼA��G�dz��л�ѧ���������ʣ�����AΪ��ɫ���壬EΪ��ɫ���嵥�ʣ�B��C��D��Ϊ��ɫ���壬FΪʯ��ʯ����Ҫ�ɷ֣�GΪ��ɫ��Һ���������ͼ�ش��������⣺
��ͼA��G�dz��л�ѧ���������ʣ�����AΪ��ɫ���壬EΪ��ɫ���嵥�ʣ�B��C��D��Ϊ��ɫ���壬FΪʯ��ʯ����Ҫ�ɷ֣�GΪ��ɫ��Һ���������ͼ�ش��������⣺