��Ŀ����
��1����H��O��C��Cl��Na��Ca����Ԫ����ѡ���ʵ�Ԫ�أ���ɷ�������Ҫ������ʣ������л�ѧʽ����ո��С�
���˹������������ ��
�ڿ����ڽ������������� ��
�ۿ��������ͷۺ�����θ�����֢���� ��
�ܿ����������ϵ��� ��
��2���˵�θҺ�к������������ᡣ��Ҫ���йصĻ�ѧ����ʽ��д�ں����ϡ�
��θ�����ʱ�������ú�������������ҩ���������___________________________��
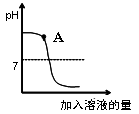
��Ba2+�ж�����X�����θ��ʱ�����ñ��ͣ����ᱵ���������̼�ᱵ�������ж������е�ԭ��Ϊ ��
�����̼�ᱵ�ж������������кҩ������þ�����ⶾ��ԭ���� ��
��3���ܽ�ͬѧ���˸������ͣ�����һ�����ʡ���Ƥ������ӡ����ͼ��ʾ�̱꣬���ᵽһ�ɴ̼�����ζ���ܽ���˸�����˵��
����������ڻ����е� ������ţ���
A.�ط� B.���� C.��
ÿ���û����к���Ԫ�ص���������Ϊ kg��
�������Ӧ���е������� ������ţ���
A.������ˮ B.�лӷ��� C.�����ֽ�
�����������ǿ�Ӧ���������ռӦ�ķ�Ӧ����ʽΪ��
NH4NO3 + NaOH �� NaNO3 + NH3�� + X����X�Ļ�ѧʽΪ ��ʩ�ø������ʱ��Ҫ������ ��ѡ��ᡱ��������ʻ��ã�����ή�ͷ�Ч��
��1��CO2 HCl NaHCO3 CaCO3
��2����Al(OH)3+3HCl=AlCl3+3H2O
��BaCO3+2HCl= BaCl2+H2O+ CO2��
��BaCl2+MgSO4=BaSO4��+MgCl2
��3����B 17.15 ��AC ��H2O ��
����������������ȸ�������ȷ�����ʵĻ�ѧ���ƣ�Ȼ�������Ŀ���ṩ��Ԫ�ء���д��ѧʽ�ķ����Ͳ���д�����ʵĻ�ѧʽ���ɣ���1���ٸɱ�������Ķ�����̼������ʱ���մ������ȣ��������˹����꣬�仯ѧʽΪ��CO2���ڽ�������������ǽ���������������ᷴӦ�������ṩ��Ԫ�ؿ�֪���������Ҫ���仯ѧʽΪ��HCl����̼�����ƿ��������ͷۺ�����θ�����֢���仯ѧʽΪ��NaHCO3����̼����ǿ����������ϵ��Σ��仯ѧʽΪ��CaCO3��
��2����д��ѧ����ʽʱ��������Ϥ��Ӧ�������ͷ�Ӧ�������������ݿ���ʵ�����������غ㶨�ɣ����������������ᷴӦ�����Ȼ�����ˮ�� Al��OH��3+3HCl�TAlCl3+3H2O��
��̼�ᱵ�����ᷴӦ���ɿ�����ˮ���Ȼ�����ˮ�Ͷ�����̼��BaCO3+2HCl�TBaCl2+CO2��+H2O��
�ۿ�����ˮ���Ȼ���������þ��Ӧ�������ᱵ�������Ȼ�þ��MgSO4+BaCl2�TBaSO4��+MgCl2��
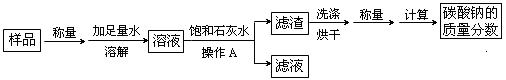
��3�����ݱ�ǩ��֪��������炙�ѧʽ�к��е�Ԫ�أ����ڵ��ʣ�ÿ�������к���Ԫ�ص���������Ϊ��50kg��34.3%=17.15kg��
�ڸ���������Ϣ��֪������״�������������ﴦ����˸û���������ˮ������ʱ���ֽ⣻
����NH4NO3+NaOH=NaNO3+NH3��+X�������غ㶨�ɿ�֪��ÿ��X�к���2����ԭ�Ӻ�1����ԭ�ӣ���ˮ��������ܺ��������Ʒ�Ӧ���ɰ���������ʩ�ø������ʱ��Ҫ������������ʻ��ã�����ή�ͷ�Ч��
���㣺��ѧʽ����д�����壻��д��ѧ����ʽ�����ֱ���ʽ�����뷽��ʽ����ǩ�ϱ�ʾ�����ʳɷּ��京�����������ʵ���������ã��̬���ʵļ��飻�����غ㶨�ɼ���Ӧ��