��Ŀ����
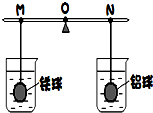 �ڸܸ˵����˹����������������ͬ�������������ʱ�ܸ�ƽ�⣬������ֱ������������ͬ��������������Ҳ��ͬ��ϡ�����У���ͼ��ʾ��ֱ�������ձ��о�û�����ݲ���Ϊֹ����������α仯�����������֣������Ʋ�����ȷ���ǣ����������ϸ��ŵ�Һ����Բ��ƣ���ͬ����������
�ڸܸ˵����˹����������������ͬ�������������ʱ�ܸ�ƽ�⣬������ֱ������������ͬ��������������Ҳ��ͬ��ϡ�����У���ͼ��ʾ��ֱ�������ձ��о�û�����ݲ���Ϊֹ����������α仯�����������֣������Ʋ�����ȷ���ǣ����������ϸ��ŵ�Һ����Բ��ƣ���ͬ���������������������������������������ͬ�������������ܶȴ�ܶ࣬��������һ���ǿ��ĵģ�������ֱ������������ͬ��Ũ����ͬ��ϡ�����У������ڷ�Ӧ���������������α仯�����������֣�˵����������ʣ�࣬������ȫ��Ӧ��ͨ����ѧ����ʽ�����֪��ͬ����������������������Ҫ�����࣬���Է�Ӧ������Ҫ�������ᣬ��֧��Ӧ��N�ƶ���
����⣺ͨ����ѧ����ʽ������������������ڵ������ȣ�
�⣺�����ĵ��������������ֱ�Ϊx��y�����������Ϊ100g����������Ϊa%��
Fe+H2SO4=FeSO4+H2�������� 2Al+3H2SO4=Al2��SO4��3+3H2��
56����98�������������������������� ��54����294
x����100g��a%������������������ ���� y����100g��a%
��
=
��ã�x=
g
=
��ã�y=
g
�����ĵ�������������֮��Ϊ��
g��
g=28��9
A���������ȿɼ���������ٵ�����������֧��Ӧ��N�ƶ����ʴ������
B�������������������������ͬ�������������ܶȴ�ܶ࣬�������ǿ��ĵģ���������һ���ǿ��ĵģ�����ȷ��
C����Ϊ������ٵ������������õ��ձ���ܸ���������б���ʴ������
D����Ϊ������ٵ����������������Ǹ��ձ�����Һ�������ӵĶ࣬������ȷ��
��ѡBD��
�⣺�����ĵ��������������ֱ�Ϊx��y�����������Ϊ100g����������Ϊa%��
Fe+H2SO4=FeSO4+H2�������� 2Al+3H2SO4=Al2��SO4��3+3H2��
56����98�������������������������� ��54����294
x����100g��a%������������������ ���� y����100g��a%
��
| 56 |
| x |
| 98 |
| 100g��a% |
| 56a |
| 98 |
| 54 |
| y |
| 294 |
| 100g��a% |
| 54a |
| 294 |
�����ĵ�������������֮��Ϊ��
| 56a |
| 98 |
| 54a |
| 294 |
A���������ȿɼ���������ٵ�����������֧��Ӧ��N�ƶ����ʴ������
B�������������������������ͬ�������������ܶȴ�ܶ࣬�������ǿ��ĵģ���������һ���ǿ��ĵģ�����ȷ��
C����Ϊ������ٵ������������õ��ձ���ܸ���������б���ʴ������
D����Ϊ������ٵ����������������Ǹ��ձ�����Һ�������ӵĶ࣬������ȷ��
��ѡBD��
�������ܸ˻���ƽƽ�����⾭���������п����ڣ����������Ҫ���ݷ�Ӧ����ȷ�ж����������ı仯��

��ϰ��ϵ�д�
�����Ŀ

 15����ͼ��ʾ���ڸܸ˵����˷ֱ����������ͬ��ͭ���п����ʱ�ܸ�ƽ�⣮Ȼ������ֱ��û��ϡH2SO4��CuSO4��Һ��һ��ʱ�䣮
15����ͼ��ʾ���ڸܸ˵����˷ֱ����������ͬ��ͭ���п����ʱ�ܸ�ƽ�⣮Ȼ������ֱ��û��ϡH2SO4��CuSO4��Һ��һ��ʱ�䣮 10���ڸܸ˵����˷ֱ���ŵ�����������������������ʱ�ܸ�ƽ�⣬������ֱ������������ȫ��ͬ��ϡ�����У�ֱ�������ձ��о������ݲ���Ϊֹ���������״�仯�������ն����֣�����ʵ�������Ʋ⣺
10���ڸܸ˵����˷ֱ���ŵ�����������������������ʱ�ܸ�ƽ�⣬������ֱ������������ȫ��ͬ��ϡ�����У�ֱ�������ձ��о������ݲ���Ϊֹ���������״�仯�������ն����֣�����ʵ�������Ʋ⣺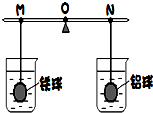 �ڸܸ˵����˹����������������ͬ�������������ʱ�ܸ�ƽ�⣬������ֱ������������ͬ��������������Ҳ��ͬ��ϡ�����У���ͼ��ʾ��ֱ�������ձ��о�û�����ݲ���Ϊֹ����������α仯�����������֣������Ʋ�����ȷ���ǣ����������ϸ��ŵ�Һ����Բ��ƣ���ͬ��
�ڸܸ˵����˹����������������ͬ�������������ʱ�ܸ�ƽ�⣬������ֱ������������ͬ��������������Ҳ��ͬ��ϡ�����У���ͼ��ʾ��ֱ�������ձ��о�û�����ݲ���Ϊֹ����������α仯�����������֣������Ʋ�����ȷ���ǣ����������ϸ��ŵ�Һ����Բ��ƣ���ͬ��