��Ŀ����
����Ŀ����һ�����İ�ɫ�����Ȼ�þ�к��������Ȼ��ơ���ѧ��ȤС���ͬѧΪ�˲ⶨ�ù����Ȼ�þ���Ȼ��Ƶ���������������������ʵ�飺����10g��Ʒ�����ձ��У������м���90gˮ�����裬ʹ����ȫ�ܽ�Ϊֹ��Ȼ��ȡ����Һһ�룬�����еμ�10%������������Һ����������������������������������Һ��������ϵ������ͼ��ʾ��
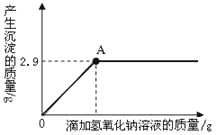
�Լ��㣺������������С����1λ��
��1����Ʒ���Ȼ�þ��������
��2��������10%������������Һ��A��ʱ��������Һ���Ȼ��Ƶ�����������
���𰸡���1��2.9g ��2��7%
��������������������ȸ�������ͼ�ɵ�Mg(OH)2������=2.9g��Ȼ����ݻ�ѧ����ʽ��MgCl2��2NaOH ==2NaCl��Mg(OH)2����Mg(OH)2��MgCl2��NaOH�Լ�����NaCl��������ϵ���ɷֱ����MgCl2��NaOH�Լ�����NaCl��������ϵ��Ȼ���һ�����㵱����10%������������Һ��A��ʱ��������Һ���Ȼ��Ƶ���������
�⣺��MgCl2����Ϊx���μӷ�Ӧ��NaOH������Ϊy�����ɵ�NaCl������Ϊz
MgCl2��2NaOH ==2NaCl��Mg(OH)2��
95 80 117 58
x y z 2.9g
(1) 95��58= x��2.9g x=4.75 g
������ȡ����Һһ������Ӧ������Ʒ��MgCl2��������4.75g��2=9.5 g
(2)ԭ��Ʒ��NaCl������=(10��4.75��2)="0.5" g
80��58==y��2.9 y=4g
������������Һ������=4g��10%=40g
117:58=z��2.9g z=5.85g
��Һ��NaCl��������=5.85g ��0.25g ��6.1g
A��ʱ��������Һ��NaCl����������=6.1g��(50g��40g��2.9g)��100%��7%

 �Ǽ�����������ϵ�д�
�Ǽ�����������ϵ�д�