��Ŀ����
��16�֣��п���ѧʵ�鿼������ѧ����ǩȷ��һ��ʵ����п��飬�����ʵ���У�����������ȡ�����ʣ��ڶ�����̼����ȡ�����ʣ�������50g15%��NaCl��Һ�����Ȱ˸�����ʵ�顣
��1����ͬѧ��ǩ������ʵ���ң���������ʵ�����ϵ���Ҫ��������������ҩƷ��
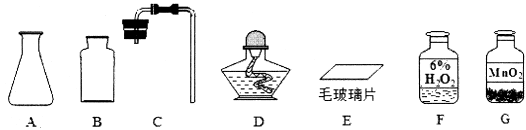
�ټ�ͬѧ�鵽��ʵ��Ӧ���� ������ĸ���ţ���
A����������ȡ������ B��������̼����ȡ������
�ڸ�ʵ�鲻���õ��������� ��д�������ƣ�����ȡ������Ļ�ѧ����ʽΪ�� ��
�ۼ�ͬѧȡ�ù���ҩƷ���뷴Ӧ�����еIJ����� ��
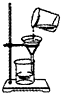
�������������巢��װ�û�������ȡ ���壬�� ��ѡ����ϡ������¡����ſ������ռ������塣
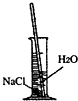
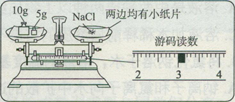
��2����ͬѧ�鵽������50g15%%��NaCl��Һ����ʵ�顣ͨ�����㣬����Ҫ����NaCl
g����ȡˮ mL����ȡˮʱ����������Ͳ��ˮ�ӽ���������ʱ��Ӧ������һ�������� ��
��1����ͬѧ��ǩ������ʵ���ң���������ʵ�����ϵ���Ҫ��������������ҩƷ��

�ټ�ͬѧ�鵽��ʵ��Ӧ���� ������ĸ���ţ���
A����������ȡ������ B��������̼����ȡ������
�ڸ�ʵ�鲻���õ��������� ��д�������ƣ�����ȡ������Ļ�ѧ����ʽΪ�� ��
�ۼ�ͬѧȡ�ù���ҩƷ���뷴Ӧ�����еIJ����� ��
�������������巢��װ�û�������ȡ ���壬�� ��ѡ����ϡ������¡����ſ������ռ������塣
��2����ͬѧ�鵽������50g15%%��NaCl��Һ����ʵ�顣ͨ�����㣬����Ҫ����NaCl
g����ȡˮ mL����ȡˮʱ����������Ͳ��ˮ�ӽ���������ʱ��Ӧ������һ�������� ��
��1����A �ھƾ��� 2H2O2 MnO2 2H2O + O2�� ���Ȱ���ƿ��ţ���ʢ�ж������̵�ҩ�ף���ֽ�ۣ�С�ĵ��͵���ƿ�ײ���Ȼ��ʹ��ƿֱ��������CO2 ���ϣ�2��7.5 42.5 ���ý�ͷ�ι���μ�ˮ������̶ȣ�����42.5mL��
�����������1����ʵ�����ϵ�ҩƷΪ��������Ͷ������̣�����ӦΪ��������ȡ���ռ���
���ù�������Ͷ���������ȡ����ʱ���ڳ����½��еģ����Բ���Ҫ�ƾ��ơ���Ӧ�Ļ�ѧ����ʽΪ��2H2O2MnO22H2O + O2��
�۱�ʵ���õ��Ĺ���ҩƷΪ�������̣���ʵ��ķ�Ӧ����Ϊ��ƿ�����Լ���ķ���Ϊ���Ȱ���ƿ��ţ���ʢ�ж������̵�ҩ�ף���ֽ�ۣ�С�ĵ��͵���ƿ�ײ���Ȼ��ʹ��ƿֱ������
�ܱ�װ��Ϊ�����Һ���ڳ�������ȡ�����װ�ã����Ի�������ʯ��ʯ��ϡ���ᷴӦ��ȡ������̼���塣������̼���ܶȱȿ��������Կ����������ſ������ռ���
��2�����������֪����Ҫ���Ȼ��Ƶ�����Ϊ50g��15%=7.5g����Ҫ��ˮ������Ϊ50g-7.5g="42.5g" ������Ҫˮ�����Ϊ��42.5g��1g/ml=42.5ml
�������ˮ������ӽ�ָ���̶�ʱ��Ҫ���ý�ͷ�ι���μ�ˮ������̶ȡ�

��ϰ��ϵ�д�
 Сѧ��10���ӿ������100��ϵ�д�
Сѧ��10���ӿ������100��ϵ�д�
�����Ŀ