��Ŀ����
��2007?��������ģ��С���ú�������������̼��һ����̼���壬����ȡ�����������һ����̼���ٻ�ԭ����������������֤��Ӧ��������ͼ��ʾʵ��װ�ûش��������⣨����A����ͼ��ʾʢ��CO2��CO�������Ĵ���ƿ����
��1��װ�����ӵ���ȷ˳����
��2��Dװ�����ܹ۲쵽��������
��3��Cװ�õ�������
��4��Eװ�õ�ȼβ����ԭ����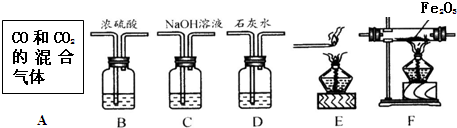
��1��װ�����ӵ���ȷ˳����
A��C��B��F��D��E
A��C��B��F��D��E
��������ţ���2��Dװ�����ܹ۲쵽��������
ʯ��ˮ�����
ʯ��ˮ�����
����װ������������Ӧ�Ļ�ѧ����ʽ��Ca��OH��2+CO2=CaCO3��+H2O
Ca��OH��2+CO2=CaCO3��+H2O
����3��Cװ�õ�������
��ȥ��������е�CO2
��ȥ��������е�CO2
����4��Eװ�õ�ȼβ����ԭ����
��ֹCO��Ⱦ����
��ֹCO��Ⱦ����
��
��������1����������˳��ȥ������̼�����﴿����һ����̼��CO���廹ԭ����ͭ��������������������ʡ���β����
��2������һ����̼��ԭ����ͭ���ж�����̼���ɽ��
��3�����������������������̼��Ӧ��֪ʶ�������⣻
��5����֪��������壬����Ҫ�ɷ�ΪCO����β����һ������δ��Ӧ��һ����̼�����������������Ⱦ������
��2������һ����̼��ԭ����ͭ���ж�����̼���ɽ��
��3�����������������������̼��Ӧ��֪ʶ�������⣻
��5����֪��������壬����Ҫ�ɷ�ΪCO����β����һ������δ��Ӧ��һ����̼�����������������Ⱦ������
����⣺��1������ʵ��Ŀ�ģ���������˳��Ϊ������������ȥ������̼�����﴿����һ����̼��CO���廹ԭ����ͭ��������������������ʡ���β����
��2��һ����̼��ԭ����ͭ���ɵĶ�����̼��ʹʯ��ˮ����ǣ���Ӧ�Ļ�ѧ����ʽΪ��CO2+Ca��OH��2�TCaCO3��+H2O��
��3��װ��C��������������Һ�����ܺͶ�����̼��Ӧ���Ӷ������ն�����̼����������̼�����ã�
��4����������к����ж���һ����̼����Eװ�õ�ȼβ����ԭ���Ƿ�ֹһ����̼��Ⱦ������
�ʴ�Ϊ����1��A��C��B��F��D��E
��2��ʯ��ˮ����ǣ�Ca��OH��2+CO2=CaCO3��+H2O
��3����ȥ��������е�CO2 ��4����ֹCO��Ⱦ����
��2��һ����̼��ԭ����ͭ���ɵĶ�����̼��ʹʯ��ˮ����ǣ���Ӧ�Ļ�ѧ����ʽΪ��CO2+Ca��OH��2�TCaCO3��+H2O��
��3��װ��C��������������Һ�����ܺͶ�����̼��Ӧ���Ӷ������ն�����̼����������̼�����ã�
��4����������к����ж���һ����̼����Eװ�õ�ȼβ����ԭ���Ƿ�ֹһ����̼��Ⱦ������
�ʴ�Ϊ����1��A��C��B��F��D��E
��2��ʯ��ˮ����ǣ�Ca��OH��2+CO2=CaCO3��+H2O
��3����ȥ��������е�CO2 ��4����ֹCO��Ⱦ����
���������⿼��һ����̼�Ͷ�����̼�����ʺͼ��鷽�����������������ӷ�����ѧ�������������������ʵķ�����

��ϰ��ϵ�д�
 ���Ǽ���С����ϵ�д�
���Ǽ���С����ϵ�д� �Ͻ�ƽ���Ȿϵ�д�
�Ͻ�ƽ���Ȿϵ�д�
�����Ŀ