��Ŀ����
��1�����������Ҫ�ɷ��ǣ�
��2��д��CO��Fe2O3�ڸ��������·�Ӧ�Ļ�ѧ����ʽ��
��3����¯�����õ�����Ҫ��Ʒ��
��4����ҵ�ϻ���ͭ��һ����Ҫ��Ӧ��Cu2S+O2
2Cu+SO2��Cu2S��ͭԪ�ص���������Ϊ
Fe3O4
Fe3O4
�����ѧʽ����2��д��CO��Fe2O3�ڸ��������·�Ӧ�Ļ�ѧ����ʽ��
3CO+Fe2O3
2Fe+3CO2
| ||
3CO+Fe2O3
2Fe+3CO2
��
| ||
��3����¯�����õ�����Ҫ��Ʒ��
B
B
��������ţ� A������ B������ C������4����ҵ�ϻ���ͭ��һ����Ҫ��Ӧ��Cu2S+O2
| ||
80%
80%
����500�ֺ�Cu2S 80%��ͭ��ʯ�������Ͽ�������320
320
��ͭ����������1�����������Ҫ�ɷ��ǣ�������������
��2���������֪����Ӧ��Ϊһ����̼������������Ӧ����Ϊ���£�������Ϊ���������̼��
��3����¯�����õ�����Ҫ��Ʒ�����Ļ����-----����������̼Ԫ�أ���
��4�����ݻ�ѧʽCu2S����ͭԪ�ص�����������������һ��������Cu2S������ͭԪ�ص�������������ijԪ�ص��������������Ǹ�Ԫ�ص�������������ʵ�Ԫ��������֮�ȣ�
��2���������֪����Ӧ��Ϊһ����̼������������Ӧ����Ϊ���£�������Ϊ���������̼��
��3����¯�����õ�����Ҫ��Ʒ�����Ļ����-----����������̼Ԫ�أ���
��4�����ݻ�ѧʽCu2S����ͭԪ�ص�����������������һ��������Cu2S������ͭԪ�ص�������������ijԪ�ص��������������Ǹ�Ԫ�ص�������������ʵ�Ԫ��������֮�ȣ�
����⣺��1�����������Ҫ�ɷ��ǣ��������������仯ѧʽΪFe3O4��
��2���������֪��Ӧ���Ӧ�������������д��CO��Fe2O3�ڸ��������·�Ӧ�Ļ�ѧ����ʽ��Fe2O3+3CO
2Fe+3CO2��
��3����¯�����õ�����Ҫ��Ʒ�����Ļ����-------����B��
��4��Cu2S��ͭԪ�ص���������=
��100%=80%����500�ֺ�Cu2S 80%��ͭ��ʯ�к�ͭԪ������=500t��80%��80%=320t��
�ʴ�Ϊ����1��Fe3O4����2��Fe2O3+3CO
2Fe+3CO2����3��B����4��80%��320��
��2���������֪��Ӧ���Ӧ�������������д��CO��Fe2O3�ڸ��������·�Ӧ�Ļ�ѧ����ʽ��Fe2O3+3CO
| ||
��3����¯�����õ�����Ҫ��Ʒ�����Ļ����-------����B��
��4��Cu2S��ͭԪ�ص���������=
| 64��2 |
| 160 |
�ʴ�Ϊ����1��Fe3O4����2��Fe2O3+3CO
| ||
������������Ҫ����ѧ��������ѧ��ѧ֪ʶ�ۺϷ����ͽ��ʵ�������������������ѧ�����������˼ά��ȣ�ǿ����ѧ������֪ʶ��������

��ϰ��ϵ�д�
�����Ŀ
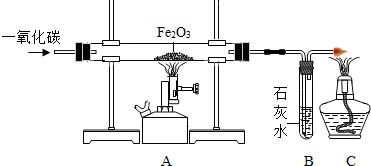
 ��2013?�����ģ�⣩�����Ƿdz���Ҫ�Ľ������ϣ��������������ǻ��������Ĵ�������Ҫ�ɷ�ΪFe3O4�����ܴ����ﵽ4.34�ڶ֣�ʵ���ҿ�����ͼװ��ģ�ҵ������
��2013?�����ģ�⣩�����Ƿdz���Ҫ�Ľ������ϣ��������������ǻ��������Ĵ�������Ҫ�ɷ�ΪFe3O4�����ܴ����ﵽ4.34�ڶ֣�ʵ���ҿ�����ͼװ��ģ�ҵ������