��Ŀ����
 ��NaOH��Na2CO3�����l8.6g��Ϊ�ⶨ����NaOH������������������ˮ��������ϡ������100gʱ����ʼ�������壻�ټ���ϡ������100gʱ�����ٲ������壬�������干4.4g
��NaOH��Na2CO3�����l8.6g��Ϊ�ⶨ����NaOH������������������ˮ��������ϡ������100gʱ����ʼ�������壻�ټ���ϡ������100gʱ�����ٲ������壬�������干4.4g����ʾ��Na2CO3+H2SO4=Na2SO4+CO2��+H2O��
��1����ͼ�л����������������������ϡ���������Ĺ�ϵ���ߣ�
��2����������NaOH�������Ƕ��٣�
��3������ϡ������������������Ƕ��٣�
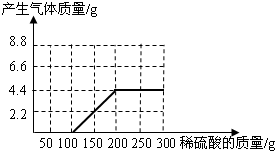
��������1�������������֪��������100g����֮��ſ�ʼ����������̼�����ɶ�����̼���������Ϊ4.4g���������������Ϊ200g�����Ծݴ���ɸ���Ľ��
��2�������������ɶ�����̼���������Ϊ4.4g�����Կ��Ը��ݻ�ѧ����ʽ�����̼���Ƶ�������Ȼ�������������Ƶ�������
��3�����ݶ�����̼����������������100gϡ������������������������ϡ���������������
��2�������������ɶ�����̼���������Ϊ4.4g�����Կ��Ը��ݻ�ѧ����ʽ�����̼���Ƶ�������Ȼ�������������Ƶ�������
��3�����ݶ�����̼����������������100gϡ������������������������ϡ���������������
����⣺��1��������100g����֮��ſ�ʼ����������̼�����ɶ�����̼���������Ϊ4.4g���������������Ϊ200g�����Կ��Ի������������������������ϡ����������Ĺ�ϵ���ߣ�����ͼ��ʾ����
��2����������Na2CO3������Ϊx��100g������Һ��H2SO4������Ϊy��
Na2CO3+H2SO4�TNa2SO4+H2O+CO2��
106 98 44
x y 4.4g
=
=
��ã�x=10.6g y=9.8g��
��2����������������Ƶ�����Ϊ18.6g-10.6g=8.0g
��3��ϡ�����������������Ϊ��
��100%=9.8%
�𣺣�2����������NaOH��������8.0g��
��3������ϡ�������������������9.8%��
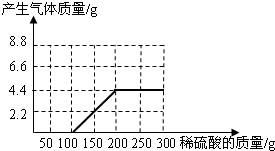
�ʴ�Ϊ����1��������ͼ��
��2��8.0g��
��3��9.8%��

��2����������Na2CO3������Ϊx��100g������Һ��H2SO4������Ϊy��
Na2CO3+H2SO4�TNa2SO4+H2O+CO2��
106 98 44
x y 4.4g
| 106 |
| x |
| 98 |
| y |
| 44 |
| 4.4g |
��ã�x=10.6g y=9.8g��
��2����������������Ƶ�����Ϊ18.6g-10.6g=8.0g
��3��ϡ�����������������Ϊ��
| 9.8g |
| 100g |
�𣺣�2����������NaOH��������8.0g��
��3������ϡ�������������������9.8%��
�ʴ�Ϊ����1��������ͼ��

��2��8.0g��
��3��9.8%��
������������Ҫ��������εĻ�ѧ���ʵĻ����ϣ��������������������ļ������⣬ע��ѧ���ۺ����������������п��Ŀ���֮һ��

��ϰ��ϵ�д�
 �߽�������ϵ�д�
�߽�������ϵ�д�
�����Ŀ


