��Ŀ����
23���������ǶԶ�����̼�й��������̽����
��1��ֲ����й������ʱ���費�����Ŀ����еĶ�����̼����������̼�ڴ����еĺ���ȴ�������ֲ��䣬��������
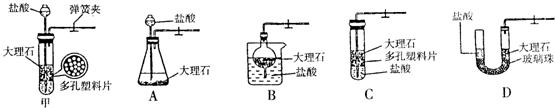
��2��ơ�ơ����֡�ѩ�̵��������г��������ϣ����к��ж�����̼��ˮ�����ʣ������������ʵ�鷽������ƿװơ�ƣ�����֡�ѩ�̵ȣ��еĶ�����̼��
����һ��
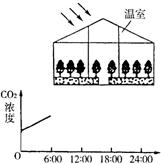
��3����ͼ��ʾ��һ���ִ���ֲ���������䣨���ң���ֲ����������Ҫ�����ʿ�ͨ�����ڶ�����̼�����䣮
������������У����˹����Ƶ�Ӫ��Һ���������߲ˣ�������ֲ����¶ȼ�������Ԫ�ص�����Ҫʵ��ͨ������CO2ʹ�����������������һ������
�ڴ����ҿ���Ϊһ��С�͵���̬ϵͳ�����ɸ�ϵͳ����������

��1��ֲ����й������ʱ���費�����Ŀ����еĶ�����̼����������̼�ڴ����еĺ���ȴ�������ֲ��䣬��������
�٢ڢۢ�
��������ţ��ٶ�ֲ��ĺ������ã��ڻ�ʯȼ�ϵ�ȼ�գ���������ֲ��ĸ��ã���ijЩ������CaCO3�ȵķֽ⣮��2��ơ�ơ����֡�ѩ�̵��������г��������ϣ����к��ж�����̼��ˮ�����ʣ������������ʵ�鷽������ƿװơ�ƣ�����֡�ѩ�̵ȣ��еĶ�����̼��
����һ��
��ơ���в�������ͨ��ʯ��ˮ��
����ѧ����ʽΪ��CO2+Ca��OH��2=CaCO3��+H2O
������������ơ���в�������ͨ�����ʯ���ˮ��
_����ѧ����ʽΪ��H2O+CO2=H2CO3
����3����ͼ��ʾ��һ���ִ���ֲ���������䣨���ң���ֲ����������Ҫ�����ʿ�ͨ�����ڶ�����̼�����䣮

������������У����˹����Ƶ�Ӫ��Һ���������߲ˣ�������ֲ����¶ȼ�������Ԫ�ص�����Ҫʵ��ͨ������CO2ʹ�����������������һ������
����
���ڴ����ҿ���Ϊһ��С�͵���̬ϵͳ�����ɸ�ϵͳ����������
ֲ��߲ˣ�
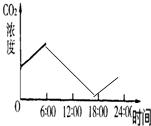
��������̬ϵͳ��ֻ�������ߣ��ڲ�ͨ��CO2������£�ÿ��ֻ��6��00��18��00�����չ����䣬������ͼ����������Ǧ�ʻ���6��00��24��00��������CO2Ũ�ȵı仯���ߣ�0��00��6��00�������Ѿ��������
��������Ȼ���м��в���������̼��;�����������Ķ�����̼��;�������������غ㶨�ɿ�����д��ѧ����ʽ������ʵ��������ȷ�Ļ���ͼ�⣮
��� �⣺��1������Ȼ���в���������̼��;���У��ٶ�ֲ��ĺ������ã��ڻ�ʯȼ�ϵ�ȼ�գ���������ֲ��ĸ��ã���ijЩ������CaCO3�ȵķֽ⣮
�⣺��1������Ȼ���в���������̼��;���У��ٶ�ֲ��ĺ������ã��ڻ�ʯȼ�ϵ�ȼ�գ���������ֲ��ĸ��ã���ijЩ������CaCO3�ȵķֽ⣮
����٢ڢۢܣ�
��2������һ����ơ���в�������ͨ��ʯ��ˮ�У�
��Ӧ�Ļ�ѧ����ʽΪ��CO2+Ca��OH��2=CaCO3��+H2O��
����������ơ���в�������ͨ�����ʯ���ˮ�У�
��Ӧ�Ļ�ѧ����ʽΪ��H2O+CO2=H2CO3
��3����һ�����ǹ��գ�������գ�
��������ֲ��߲ˣ������ֲ��߲ˣ���
 �⣺��1������Ȼ���в���������̼��;���У��ٶ�ֲ��ĺ������ã��ڻ�ʯȼ�ϵ�ȼ�գ���������ֲ��ĸ��ã���ijЩ������CaCO3�ȵķֽ⣮
�⣺��1������Ȼ���в���������̼��;���У��ٶ�ֲ��ĺ������ã��ڻ�ʯȼ�ϵ�ȼ�գ���������ֲ��ĸ��ã���ijЩ������CaCO3�ȵķֽ⣮����٢ڢۢܣ�
��2������һ����ơ���в�������ͨ��ʯ��ˮ�У�
��Ӧ�Ļ�ѧ����ʽΪ��CO2+Ca��OH��2=CaCO3��+H2O��
����������ơ���в�������ͨ�����ʯ���ˮ�У�
��Ӧ�Ļ�ѧ����ʽΪ��H2O+CO2=H2CO3
��3����һ�����ǹ��գ�������գ�
��������ֲ��߲ˣ������ֲ��߲ˣ���
������������Ҫ�����˻�ѧ����ʽ����д����Ȼ�����������̼��;��������ȷ��ͼ�ȷ�������ݣ�

��ϰ��ϵ�д�
 �¿α�����Ķ�ѵ��ϵ�д�
�¿α�����Ķ�ѵ��ϵ�д�
�����Ŀ