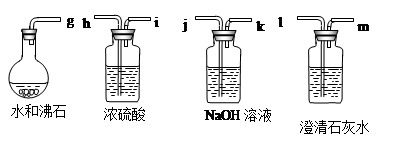��Ŀ����
���ǵ��ճ������벻��������
��1���� ��������������е�ˮ������_ �ȷ�����ѧ��Ӧ�Ĺ��̡�
��2����̽������ͭ��п�����Ľ������˳��ʱ��ijС��������������ʵ�飺
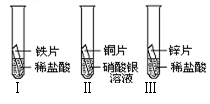
�����ý�������״���С��ϡ�������������ͬ��
��һ��ʱ��ɹ۲쵽ʵ���������� ��
��Ӧ�Ļ�ѧ����ʽ��
��С���еļ�ͬѧ��Ϊ��ͨ��ʵ���͢�ɱȽϳ�п�����Ľ������ǿ��������Ϊ�����ݵ�ʵ�������� ��
���Һͱ���λͬѧ��Ϊ��������ʵ�鲻�ܹ��ó����ֽ����Ļ��˳��ԭ��
�� ��
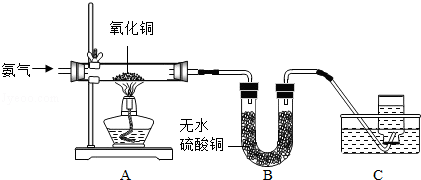
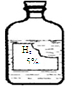
��������ʵ��Ļ����ϣ�������һ��ʵ�飨��ͼ��ʾ����ʵ����̽��Ŀ�ġ����ǵ�ʵ�飺X�ǽ���______��Y��__________��Һ��д����ѧʽ����
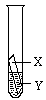
��1���� ��������������е�ˮ������_ �ȷ�����ѧ��Ӧ�Ĺ��̡�
��2����̽������ͭ��п�����Ľ������˳��ʱ��ijС��������������ʵ�飺

�����ý�������״���С��ϡ�������������ͬ��
��һ��ʱ��ɹ۲쵽ʵ���������� ��
��Ӧ�Ļ�ѧ����ʽ��
��С���еļ�ͬѧ��Ϊ��ͨ��ʵ���͢�ɱȽϳ�п�����Ľ������ǿ��������Ϊ�����ݵ�ʵ�������� ��
���Һͱ���λͬѧ��Ϊ��������ʵ�鲻�ܹ��ó����ֽ����Ļ��˳��ԭ��
�� ��
��������ʵ��Ļ����ϣ�������һ��ʵ�飨��ͼ��ʾ����ʵ����̽��Ŀ�ġ����ǵ�ʵ�飺X�ǽ���______��Y��__________��Һ��д����ѧʽ����
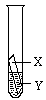
��1�� ��������O2��
��2���ٺ�ɫ���壨ͭƬ������������ɫ�������� ����ɫ��Һ����ɫ�����һ����֣��� Cu+2Ag NO3 =" Cu(" NO3)2+2 Ag
��ʵ����в�����������ʱ�ʵ���Ŀ�
�����Ƚ�Fe��Cu�Ļ�ԣ�����ȷ������ͭ�Ļ��˳��
Fe CuSO4(��Cu H2SO4����Fe SO4 Cu) �����վ���Ը��֣�
��2���ٺ�ɫ���壨ͭƬ������������ɫ�������� ����ɫ��Һ����ɫ�����һ����֣��� Cu+2Ag NO3 =" Cu(" NO3)2+2 Ag
��ʵ����в�����������ʱ�ʵ���Ŀ�
�����Ƚ�Fe��Cu�Ļ�ԣ�����ȷ������ͭ�Ļ��˳��
Fe CuSO4(��Cu H2SO4����Fe SO4 Cu) �����վ���Ը��֣�
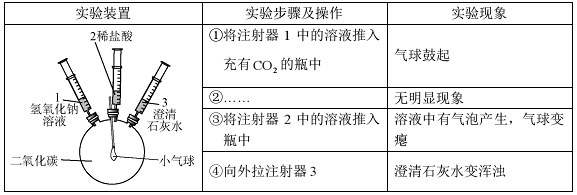
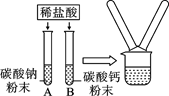
�����������1������������������ǣ�������������ˮͬʱ�Ӵ�ʱ�����������⣻
��2����ʵ����У�ͭ�Ļ�Ա���ǿ���ܹ�����������Һ���û���������ɫͭƬ����������ɫ������������ɫ��Һ����ɫ���䷴Ӧ��ѧ����ʽΪ��Cu+2AgNO3�TCu�� NO3��2+2Ag��
�ڢ�������ϡ�����ܹ���Ӧ������пƬҲ�ܹ���ϡ���ᷴӦ��������ͬʱ����Ӧ�����ǵ���״���С��ϡ�����������������������ͬ�������£�ʵ����в���������ٶȱ�ʵ���Ŀ죬п������Ӧ���ң���˵����п���������ã�
��ʵ����ʵ�����֤��п�������ǿ��ʵ���ʵ������Ƚ�Fe��Cu�Ļ�ԣ��ڲ���ʵ���У��������û�����ͭ�е�ͭ������֤������ͭ���ã��ʿ��жϳ������ֽ����Ļ����ǿ������˳��Ϊп������ͭ�����ǵĻ�Զ�����ǿ��
������������������ʼ���Ӧ�ã�ͨ��ʵ��̽�������Ļ��˳�������������ԭ������ȷ��д��ѧ����ʽ������������ѧ����̽��������ʵ������������ۺϽ�������������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ