��Ŀ����
��2013?������һģ��Ϊ�˲ⶨijƷ�ƴ������Ϊ�Ȼ��ƣ���̼���Ƶ���������������������ʵ�飺���������ձ��ж�������11g��Ʒ��������ˮ�����Һ�����������ձ��м����������ȵ�CaCl2��ĩ���۽���ַ�Ӧ�����ɵij������ˡ�ϴ�ӡ�����õ�wg�İ�ɫ���壮ʵ���������£�| �ձ��� | �ձ��� | �ձ��� | |
| ����CaCl2������/g | 5.55 | 12 | 15 |
| W/g | 5 | 10 | 10 |
��1�����ձ����е���Һ���ɣ��õ�______g���壬�ù�����______��______��ɣ�ֻ�ѧʽ����
��2����Ʒ��̼���Ƶ����������Ƕ��٣�
���𰸡�����������̽�����ʵ����������̼���ƺ��Ȼ��Ƶķ�Ӧ�����11g��Ʒ��̼���Ƶ��������ɱ������ݣ�5.55g�Ȼ��Ʋ��㣬10g��12g�Ȼ��ƶ���ʹ̼������ȫ��Ӧ�����Կɸ�����10g̼���������չ�����㣮
����⣺��1�����ݱ������ݣ�11g��Ʒ���������10g̼��ƣ���11g��Ʒ��̼���Ƶ�����ΪX�����ĵ��Ȼ���Yg��
��Ӧ���ɵ��Ȼ���Zg����
Na2CO3+CaCl2=CaCO3��+2NaCl
106 111 100 117
X Y 10g z
106��100=X��10 111��100=Y��10 100��117=10��Z
��ã�X=10.6g Y=11.1g Z=11.7g
��11g��Ʒ��NaClΪ11g-10.6g=0.4g���ձ�����ʣ���CaCl2Ϊ15g-11.1g=3.9g�����ɵ��Ȼ���Ϊ11.7g
�����ձ����е���Һ���ɣ��õ�NaCl��CaCl2
������0.4g+3.9g+11.7g=16g
�ʴ�Ϊ��16��NaCl��CaCl2
��2����Ʒ��̼���Ƶ���������Ϊ��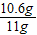 ×100%=96.4%
×100%=96.4%
�ʴ�Ϊ��96.4%
����������˼·��������Ϸ�������ȷ�������ձ��з�ӦҺ��ijɷ�ʱ��Ҫע���Ȼ����������֣�ԭ���ĺ����ɵģ�
����⣺��1�����ݱ������ݣ�11g��Ʒ���������10g̼��ƣ���11g��Ʒ��̼���Ƶ�����ΪX�����ĵ��Ȼ���Yg��
��Ӧ���ɵ��Ȼ���Zg����
Na2CO3+CaCl2=CaCO3��+2NaCl
106 111 100 117
X Y 10g z
106��100=X��10 111��100=Y��10 100��117=10��Z
��ã�X=10.6g Y=11.1g Z=11.7g
��11g��Ʒ��NaClΪ11g-10.6g=0.4g���ձ�����ʣ���CaCl2Ϊ15g-11.1g=3.9g�����ɵ��Ȼ���Ϊ11.7g
�����ձ����е���Һ���ɣ��õ�NaCl��CaCl2
������0.4g+3.9g+11.7g=16g
�ʴ�Ϊ��16��NaCl��CaCl2
��2����Ʒ��̼���Ƶ���������Ϊ��
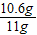 ×100%=96.4%
×100%=96.4%�ʴ�Ϊ��96.4%
����������˼·��������Ϸ�������ȷ�������ձ��з�ӦҺ��ijɷ�ʱ��Ҫע���Ȼ����������֣�ԭ���ĺ����ɵģ�

��ϰ��ϵ�д�
�����Ŀ
 ���ĵ�����Ϊ
���ĵ�����Ϊ