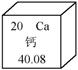
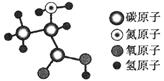
��Ŀ����
��4�֣�ͭ��������������̼�����������һ����̼�dz��л�ѧ�г��������ʣ���ͼ�����ǵIJ���Ӧ�á�

��Щ�����У���1��ͭ�������������ߣ���Ϊ�������õ���չ�Ժ�________�ԡ�
��2������������������˹�����������ǣ������ʵĻ�ѧʽ�� ��
��3����¯�����л�ԭ�������Ļ�ѧ����ʽΪ ��
��4������̽�����ú��������������ˮ�����˿�ʱ�ῴ����С���ݲ������仯ѧ����ʽΪ ��

| A������ | B����¯���� | C���ɱ� | D��ҽ������ |
��2������������������˹�����������ǣ������ʵĻ�ѧʽ�� ��
��3����¯�����л�ԭ�������Ļ�ѧ����ʽΪ ��
��4������̽�����ú��������������ˮ�����˿�ʱ�ῴ����С���ݲ������仯ѧ����ʽΪ ��
��1�������ԣ�2��CO2��3��Fe2O3+3CO 2Fe+3CO2��4��2H2O2=2H2O+O2��
2Fe+3CO2��4��2H2O2=2H2O+O2��
 2Fe+3CO2��4��2H2O2=2H2O+O2��
2Fe+3CO2��4��2H2O2=2H2O+O2�������������1������������Ҫ�����õļӹ����ܺ����õĵ����ԣ���2���������˹�����������Ǹɱ�����ѧʽΪCO2����3����¯������ԭ����һ����̼��ԭ����������һ����̼���Ǽ����̼������Ӧ�����ģ���4�������������˿�������Ĵ������������Ӷ�ɱ��ϸ����
���������������ʵ������ر��ǻ�ѧ�������п������ڱؿ����ͣ����ѣ�����Ӧע����ۣ��ر���һЩϸС�������֪ʶ�㡣

��ϰ��ϵ�д�
�����Ŀ