��Ŀ����
����5�£����и�У�����˻�ѧʵ��������飬һЩͬѧ����ͼ��ʾ��������װ�ý��г����������ȡ������ʵ�飮��ش��������⣺
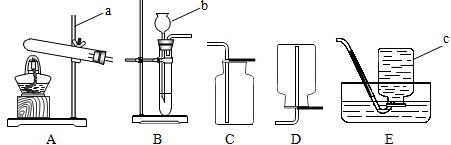
����ʵ��װ��ͼ�ش�
��1��д��ͼ�б��б�����������ƣ�
a �� b ��
��2��С��ͬѧ��ʯ��ʯ��ϡ������ȡ���ռ�һƿ������̼��Ӧѡ��ͼ���е� �� װ�ã���װ�ö�Ӧ����ĸ�����÷�Ӧ�ķ��ű���ʽ�� �ռ�������̼��С�콫ȼ�ŵ�ľ�����ڼ���ƿ�ڣ��������ռ������ɹ۲쵽�������� ��
��3��A��B����װ�þ�������ʵ������ȡ������������ ��ѡ��A��B��װ�ã����������ķ��ű���ʽΪ
��ѡװ��E�ռ����������ռ������Ǹ������� �����ʶ�ȷ���ģ�
��4��ʵ�������������ƹ�����Ȼ�粒��������ȡ�����������д̼�����ζ��������ˮ���ܶȱȿ���С����ѡ��ͼ�е� �� װ������ȡ�ռ������壮
���𰸡���������1����Ϥ�����������˽����ƣ�
��2��ʵ������ȡ����ʱҪ���ݷ�Ӧ��״̬�ͷ�Ӧ����ѡ����װ�ã����ݶ�����̼��������������
��3��ʵ������ȡ����ʱҪ���ݷ�Ӧ��״̬�ͷ�Ӧ����ѡ����װ�ã������������������ռ���
��4��ʵ������ȡ����ʱҪ���ݷ�Ӧ��״̬�ͷ�Ӧ����ѡ����װ�ã��Լ������������ѡ���ռ�װ�ã�
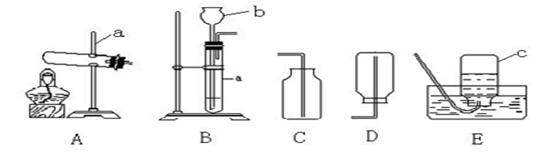
����⣺��1����Ϥ�����������˽����ƣ�a ����̨��b����©����
��2����ʯ��ʯ��ϡ������ȡ���ռ�һƿ������̼����Ӧ���ǹ����Һ���ϣ��ҷ�Ӧ����Ҫ���ȣ��ʷ���װ��ѡ��B��������̼���ܶȱȿ�������������ˮ�����ռ�װ��ѡ��C���÷�Ӧ�ķ��ű���ʽ��CaCO3+2HCl�TCaCl2+H2O+CO2����������̼��ȼ��Ҳ��֧��ȼ�գ�С�콫ȼ�ŵ�ľ�����ڼ���ƿ�ڣ��������ռ������ɹ۲쵽��������ľ��Ϩ��
��3��Aװ�������ڷ�Ӧ��Ϊ������������Ҫ���ȣ����Կ��ü��ȸ�����صķ�����ȡ��������Ӧ�Ļ�ѧ����ʽΪ��2KMnO4 K2MnO4+MnO2+O2����Bװ�������ڷ�Ӧ���ǹ����Һ���ϣ��ҷ�Ӧ����Ҫ���ȣ�����˫��ˮ�Ͷ������̻����ȡ��������Ӧ�Ļ�ѧ����ʽΪ��2H2O2
K2MnO4+MnO2+O2����Bװ�������ڷ�Ӧ���ǹ����Һ���ϣ��ҷ�Ӧ����Ҫ���ȣ�����˫��ˮ�Ͷ������̻����ȡ��������Ӧ�Ļ�ѧ����ʽΪ��2H2O2 2H2O+O2����������������ˮ����������ˮ���ռ�������
2H2O+O2����������������ˮ����������ˮ���ռ�������
��4��ʵ�������������ƹ�����Ȼ�粒��������ȡ���������Է���װ��ѡ��A����Ϊ�����д̼�����ζ��������ˮ���ܶȱȿ���С�������������ſ������ռ�������
�ʴ�Ϊ����1��a ����̨��b����©����
��2��B��C��CaCO3+2HCl�TCaCl2+H2O+CO2����ľ��Ϩ��
��3��B��2H2O2 2H2O+O2������������ˮ��
2H2O+O2������������ˮ��
��4��A��D��
���������⿼�鳣���������ȡ���ռ�װ�ã������е��⣬ֵ��ע������������������������һ��Ҫ�ڼ���ƿ�ڣ�����������Ҫ�ڼ���ƿ�ڲ���Ҫע����ߵ�����
��2��ʵ������ȡ����ʱҪ���ݷ�Ӧ��״̬�ͷ�Ӧ����ѡ����װ�ã����ݶ�����̼��������������
��3��ʵ������ȡ����ʱҪ���ݷ�Ӧ��״̬�ͷ�Ӧ����ѡ����װ�ã������������������ռ���
��4��ʵ������ȡ����ʱҪ���ݷ�Ӧ��״̬�ͷ�Ӧ����ѡ����װ�ã��Լ������������ѡ���ռ�װ�ã�
����⣺��1����Ϥ�����������˽����ƣ�a ����̨��b����©����
��2����ʯ��ʯ��ϡ������ȡ���ռ�һƿ������̼����Ӧ���ǹ����Һ���ϣ��ҷ�Ӧ����Ҫ���ȣ��ʷ���װ��ѡ��B��������̼���ܶȱȿ�������������ˮ�����ռ�װ��ѡ��C���÷�Ӧ�ķ��ű���ʽ��CaCO3+2HCl�TCaCl2+H2O+CO2����������̼��ȼ��Ҳ��֧��ȼ�գ�С�콫ȼ�ŵ�ľ�����ڼ���ƿ�ڣ��������ռ������ɹ۲쵽��������ľ��Ϩ��
��3��Aװ�������ڷ�Ӧ��Ϊ������������Ҫ���ȣ����Կ��ü��ȸ�����صķ�����ȡ��������Ӧ�Ļ�ѧ����ʽΪ��2KMnO4
 K2MnO4+MnO2+O2����Bװ�������ڷ�Ӧ���ǹ����Һ���ϣ��ҷ�Ӧ����Ҫ���ȣ�����˫��ˮ�Ͷ������̻����ȡ��������Ӧ�Ļ�ѧ����ʽΪ��2H2O2
K2MnO4+MnO2+O2����Bװ�������ڷ�Ӧ���ǹ����Һ���ϣ��ҷ�Ӧ����Ҫ���ȣ�����˫��ˮ�Ͷ������̻����ȡ��������Ӧ�Ļ�ѧ����ʽΪ��2H2O2 2H2O+O2����������������ˮ����������ˮ���ռ�������
2H2O+O2����������������ˮ����������ˮ���ռ���������4��ʵ�������������ƹ�����Ȼ�粒��������ȡ���������Է���װ��ѡ��A����Ϊ�����д̼�����ζ��������ˮ���ܶȱȿ���С�������������ſ������ռ�������
�ʴ�Ϊ����1��a ����̨��b����©����
��2��B��C��CaCO3+2HCl�TCaCl2+H2O+CO2����ľ��Ϩ��
��3��B��2H2O2
 2H2O+O2������������ˮ��
2H2O+O2������������ˮ����4��A��D��
���������⿼�鳣���������ȡ���ռ�װ�ã������е��⣬ֵ��ע������������������������һ��Ҫ�ڼ���ƿ�ڣ�����������Ҫ�ڼ���ƿ�ڲ���Ҫע����ߵ�����

��ϰ��ϵ�д�
�����Ŀ