��Ŀ����
��5�֣�����ݳ����Ľ�����֪ʶ�ش��й����⣺
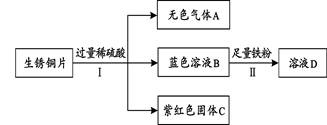
��1��д���ؿ��к������Ľ���Ԫ�صķ��� ��
��2�����Ͳ���������������������ͭ��Һ��ԭ���û�ѧ����ʽ��ʾ��_____________��
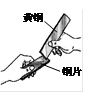
��3������ͭ����Ͻ��ڽ�ͨ�������洦�ɼ����ֽ�����Cr������Cu(NO3)2����Һ�У������к�ɫ���ʳ��֡������ͭ��ȣ��������ǿ����______________��
��4��Ŀǰ���ڶ���������������ĸ���������Ҫԭ���� ��
��5����Ҫ��ȥFeCl2��Һ�е�����CuCl2���ʣ��ɼ�������� ��
��1��д���ؿ��к������Ľ���Ԫ�صķ��� ��
��2�����Ͳ���������������������ͭ��Һ��ԭ���û�ѧ����ʽ��ʾ��_____________��
��3������ͭ����Ͻ��ڽ�ͨ�������洦�ɼ����ֽ�����Cr������Cu(NO3)2����Һ�У������к�ɫ���ʳ��֡������ͭ��ȣ��������ǿ����______________��
��4��Ŀǰ���ڶ���������������ĸ���������Ҫԭ���� ��
��5����Ҫ��ȥFeCl2��Һ�е�����CuCl2���ʣ��ɼ�������� ��
��1��Al ��2��Fe+CuSO4=Cu+FeSO4 ��3������Cr�� ��4�����ܺͿ����е�������Ӧ�������ܵ�����Ĥ���б������ã�5������
��1���ؿ��к������Ľ���Ԫ������Ԫ�أ�Ԫ�ط���ΪAl������2�����ܺ�����ͭ��Һ��Ӧ���ʲ���������������������ͭ��Һ��Ӧ�Ļ�ѧ����ʽΪ��Fe+CuSO4=Cu+FeSO4
��3��������Cr������Cu��NO3��2����Һ�У������к�ɫ���ʳ��֣�˵�����ܽ�ͭ������ͭ��Һ���û��������ʸ��Ļ�Ա�ͭǿ
��4�����Ļ�Խ�ǿ���ڳ����¿�����������Ӧ�������������γ����ܵ�Ĥ���������ı��棬�����������������ĽӴ�����ֹ�˽�һ��������
��5���������Ȼ�ͭ��Ӧ�����Ȼ�������ͭ�����˺��ֻʣ�Ȼ�������
��3��������Cr������Cu��NO3��2����Һ�У������к�ɫ���ʳ��֣�˵�����ܽ�ͭ������ͭ��Һ���û��������ʸ��Ļ�Ա�ͭǿ
��4�����Ļ�Խ�ǿ���ڳ����¿�����������Ӧ�������������γ����ܵ�Ĥ���������ı��棬�����������������ĽӴ�����ֹ�˽�һ��������
��5���������Ȼ�ͭ��Ӧ�����Ȼ�������ͭ�����˺��ֻʣ�Ȼ�������

��ϰ��ϵ�д�
�����Ŀ