��Ŀ����
����Ŀ���������ҹ�ijЩ����������Ⱦ��Ϊ���أ���Ҫ�ɴ���ȼ�պ������ߵ�ú����ɵģ�����������ŷŵ�β��Ҳ�Dz��ɺ��ӵ�һ�����أ�
��������⡿���ͨ��ʵ��֤��ú�к���̼Ԫ�غ���Ԫ�أ�
���������ϡ�I������������ʹ���������Һ��ɫ�����Ϻ�ɫ��Ϊ��ɫ�����÷�Ӧ�Ļ�ѧ����ʽΪ��5SO2+2KMnO4+2H2O=K2SO4+2MnSO4+2H2SO4 ��
II����������Ͷ�����̼һ�����ܺͳ���ʯ��ˮ������Ӧ����������ˮ��������ƣ�CaSO3����
��1����д����������ʹ����ʯ��ˮ����ǵĻ�ѧ����ʽ�� ��
��2�������������ϣ���ͬѧ����ͬѧ���ʵ�鷽������̽����
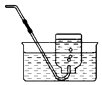
��ʵ��̽������ͬѧ��������ͼ1��ʾʵ�飨����װ����ͼ����ȥ����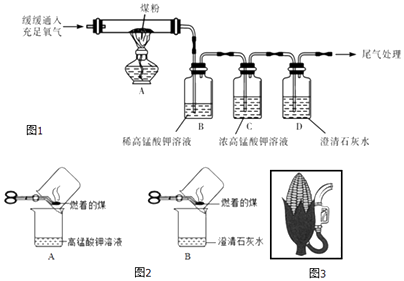
��̽������ۡ�ͼCװ�õ������� ��
��3��ʵ���п���������Щ�������֤��ú�к���̼Ԫ�غ���Ԫ�� �� ������ţ�
��B��ϡ���������Һ��ɫ��B��ϡ���������Һ����ɫ
��D�г���ʯ��ˮ����Ǣ�D�г���ʯ��ˮ�������
��4��ʵ������б�����ƺ�ͨ����������ʣ��Է�ֹ��Ӧ����ֲ���CO��һ��Ӧ�Բ���CO��ʵ��������Ҫ����β��������β�������ɲ��õķ����� ��
��5������ʵ�������ÿһ�����ܳ�ַ�Ӧ����S��C�ֱ�ת��ΪSO2��CO2 �� ������ˮ����Ӱ�죬5gú����Ʒ��ַ�Ӧ��B��C��D����װ�������ֱ�����0.8g��0.2g��13.2g�����ú����Ʒ������Ϊ�������ȷ��0.1%����
��6���������辶����ͬѧ�������ͼ2��ʾ����ʵ�飬��֤��ú�к���̼Ԫ�غ���Ԫ�أ�����Ϊ��ͬѧ������Ƿ������ �� ����ǡ��� ��ԭ���� ��
��7��Ϊ�˼���������γɣ�����ʲô�õĽ��飿 ��
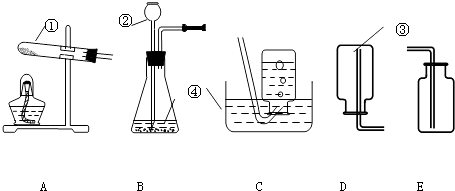
��8����ͼ3������ij��������վ�Ĺ�����������������������ж�����������ȷ���� ��
������֭����������ȼ��
�������������ľƾ���������ȼ��
���ƹ�����������ȼ�Ͽ���һ���̶��ϻ��ǰ����ԴΣ����
���𰸡�
��1��Ca��OH��2+SO2�TCaSO3��+H2O
��2����ȥ��������
��3���ۢ�
��4����ȼ���ռ�
��5��10.0%
��6��������̼�Ͷ���������ʹ����ʯ��ˮ����ǣ���ʵ�鲻��˵��úȼ�ղ���������̼
��7��ú̿��ȼ��ǰ��ȡ�����ʩ��������������Դ
��8���ڢ�
���������⣺��1���������������ʯ��ˮ��Ӧ�Ļ�ѧ����ʽΪ��Ca��OH��2+SO2�TCaSO3��+H2O����2����ͼCװ�õ������dz�ȥ������������Ӱ��Զ�����̼���жϣ������ȥ��������3������������ʹ���������Һ��ɫ��������̼ʹ����ʯ��ˮ����ǣ����B��ϡ���������Һ��ɫ��D�г���ʯ��ˮ����ǣ�����֤��ú�к���̼Ԫ�غ���Ԫ�أ���4��CO�ж���Ϊ��ֹ��Ⱦ������β�������ɲ��õķ����ǵ�ȼ����5������������ʹ���������Һ��ɫ��������̼ʹ����ʯ��ˮ����ǣ�B��Cװ�������ӵ�����Ϊ�������������Ϊ0.8g+0.2g=1g
��Ԫ�ص�����=1g�� ![]() ��100%=0.5g��
��100%=0.5g��
������Ϊ�� ![]() ��100%=10.0%����6����ͬѧ��ʵ�鷽�����������������ǣ�������̼�Ͷ���������ʹ����ʯ��ˮ����ǣ���ʵ�鲻��˵��úȼ�ղ���������̼����7��ʹ�������Դ��ú̿��ʹ�ý������������ɼ���������γɣ����ʹ�������Դ��ú̿��ʹ�ý�������������8����������Ҫ���е��ۣ���ͨ���������Ҵ����Ҵ��п�ȼ�ԣ������ȼ�ϣ�����������ȼ�ϣ����Ԣٴ������ȷ����ǰ��ԴΣ���Ӿ磬���� ���ƹ�����������ȼ�Ͽ���һ���̶��ϻ��ǰ����ԴΣ������ȷ�ģ�
��100%=10.0%����6����ͬѧ��ʵ�鷽�����������������ǣ�������̼�Ͷ���������ʹ����ʯ��ˮ����ǣ���ʵ�鲻��˵��úȼ�ղ���������̼����7��ʹ�������Դ��ú̿��ʹ�ý������������ɼ���������γɣ����ʹ�������Դ��ú̿��ʹ�ý�������������8����������Ҫ���е��ۣ���ͨ���������Ҵ����Ҵ��п�ȼ�ԣ������ȼ�ϣ�����������ȼ�ϣ����Ԣٴ������ȷ����ǰ��ԴΣ���Ӿ磬���� ���ƹ�����������ȼ�Ͽ���һ���̶��ϻ��ǰ����ԴΣ������ȷ�ģ�
���Դ��ǣ���1��Ca��OH��2+SO2�TCaSO3��+H2O����2����ȥ��������3���ۢ٣���4����ȼ���ռ���5��10.0%����6��������̼�Ͷ���������ʹ����ʯ��ˮ����ǣ���ʵ�鲻��˵��úȼ�ղ���������̼����7��ú̿��ȼ��ǰ��ȡ�����ʩ��������������Դ�ȣ����𰸺������ɣ���8���ڢۣ�
�����㾫����������Ҫ�����������غ㶨�ɼ���Ӧ�ú���д��ѧ����ʽ�����ֱ���ʽ�����뷽��ʽ�����֪ʶ�㣬��Ҫ���բ������غ㶨��ֻ�����ڻ�ѧ�仯���������������仯���ڲ��μӷ�Ӧ����������������������������������ܼ��롰�ܺ͡��У���Ҫ���ǿ����е������Ƿ�μӷ�Ӧ�����ʣ������壩������©��ע�⣺a����ƽ b������ c�����Ų�����ȷ�����⣮

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��������ʵ��ָ�������е�ˮ�������û������ˮ����Ҫ���õ��ǣ� ��
ʵ |
|
|
|
|
���� | ����ƿ�е�ˮ�����շų������� | ����ƿ�е�ˮ��ˮ������ƿ�ڵĿ����Ÿɾ������ڹ۲�������ʱ�ռ��� | ��Ͳ�е�ˮ��ͨ��ˮ����ı仯�ó�������� | ����ƿ�е�ˮ����ȴ�����ۻ����ֹ����ƿը�� |
A | B | C | D |
A.A
B.B
C.C
D.D