��Ŀ����
��2010?��������ģ��ij�Ƽ�С���ͬѧ��16������ͭ������̿�۾��Ȼ�ϣ������ͼ��ʵ��װ�ý���̽����
��ش��������⣺
������a��b�������ǣ�a______��b______��
��װ��a�е�ʵ��������______��
װ��c�з�����Ӧ�Ļ�ѧ����ʽΪ______��
��ʵ��������ܵõ�ͭ������Ϊ���٣������ݻ�ѧ����ʽ��ʽ���㣩
��______
��ͬѧ����ͨ���ⶨ����̼����Ԫ�ص����������ɶ�����̼���������Խ�һ����������ͭ��̿�۷�Ӧ�����������CO2���Ƿ������������Ӧһ��ʱ���ֹͣ���ȣ���ȴ�����£���Ӧǰ�����õ��������£�
| װ�� | ��Ӧǰ | ��Ӧ�� |
| a | �Թܵ�����36.2g ����ͭ��̿�ۻ���������Ϊ20.0g | �Թܺ������ʵ�����Ϊ54.8g |
| c | ��Ӧ��ƿ��Һ��ȷ�Ӧǰ����1.1g | |
���ڱ�֤װ�ò�©��������ȷ�������淶������װ��d�г���ʯ��ˮ������ǵ�ǰ���£�����Ϊ�����������н�����ܵ�ԭ���ǣ�______
����ͬѧ��Ϊ����ͼװ�ô�������ļ�������ͭ��̿�ۻ�����װ�ã�����ǰ��ͨһ������x��ֹͣ���Ⱥ���ͨһ������壬������ʹʵ���õ����ݸ���ȷ��˵�����⣮����Ϊ��O2��N2��H2���������У�xӦѡ���������______��
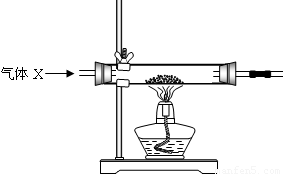
���𰸡���������1��������Ҫ����̼�Ļ�ѧ���ʣ���������ͭ��̿�ۻ�����ʵ���dz��л�ѧ��һ���ص㣬Ӧ��֪��װ�ú����ᴦ��ʵ�����ݣ���2���������������ƺ�ѡ�ã�����װ��������3�����������غ㶨�ɣ���ϻ�ѧ����ʽ�����м��㣺����δ֪��������ȷ��д��ѧ����ʽ�����ҳ���֪����δ֪�������йص���Է������������б���ʽ���ݽ����ʽ����𣻣�4��ͨ�����ݴ������ҳ�ԭ��5������ȼ��������������ȼ�Ե�����--������
����⣺������a��b�������ǣ�a�Թܣ�b�ƾ��ƣ�
��װ��a�е�ʵ�������ǣ���ɫ������ɹ�����ɫ��װ��c�з�����Ӧ�Ļ�ѧ����ʽΪ2NaOH+CO2=Na2CO3+H2O
����ʵ��������ܵõ�ͭ������Ϊx��
����2CuO+C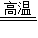 2Cu+CO2��
2Cu+CO2��
160 128
16g x
�б���ʽΪ��
x=12.8g������ͭ������Ϊ12.8�ˣ��ʴ�Ϊ12.8�ˣ�
�ܷ������ݷ��֣���Ӧ������̼����Ԫ�ص����������ڣ�����ڡ���С�ڡ����ڡ������ɶ�����̼��������
��ԭ���ǣ�װ���л���һ���� CO2δ��NaOH��Һ���գ�����ͭ��̿�۷�Ӧ���������壬��CO2���CO���𰸺������ɣ�
��xӦѡ��������ǣ�N2��������
����������������ʵ�鼼�ܣ���������������غ㶨�ɣ����ݻ�ѧ����ʽ�ļ��������̼�Ļ�ѧ���ʣ�
����⣺������a��b�������ǣ�a�Թܣ�b�ƾ��ƣ�
��װ��a�е�ʵ�������ǣ���ɫ������ɹ�����ɫ��װ��c�з�����Ӧ�Ļ�ѧ����ʽΪ2NaOH+CO2=Na2CO3+H2O
����ʵ��������ܵõ�ͭ������Ϊx��
����2CuO+C
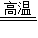 2Cu+CO2��
2Cu+CO2��160 128
16g x
�б���ʽΪ��

x=12.8g������ͭ������Ϊ12.8�ˣ��ʴ�Ϊ12.8�ˣ�
�ܷ������ݷ��֣���Ӧ������̼����Ԫ�ص����������ڣ�����ڡ���С�ڡ����ڡ������ɶ�����̼��������
��ԭ���ǣ�װ���л���һ���� CO2δ��NaOH��Һ���գ�����ͭ��̿�۷�Ӧ���������壬��CO2���CO���𰸺������ɣ�
��xӦѡ��������ǣ�N2��������
����������������ʵ�鼼�ܣ���������������غ㶨�ɣ����ݻ�ѧ����ʽ�ļ��������̼�Ļ�ѧ���ʣ�

��ϰ��ϵ�д�
 Сѧ��ʱ��ѵϵ�д�
Сѧ��ʱ��ѵϵ�д�
�����Ŀ
��2010?��������ģ����ͼ���Ǽ��ּ����������й���Ϣ��
�����ϱ��ش��������⣺
�١�����顱����ܡ����ܡ���������������ã�
�ڡ�����顱�롰Ư�������ܻ��ã�����������ײ���һ���ж����壮��Ư��������Ч�ɷ�NaClO����Ԫ�صĻ��ϼ��ǣ�
�ۡ�����顱�����ڴ���ʯ���������ǣ��û�ѧ����ʽ��ʾ����
����ʢ��������������Ư��Һ����Թ��У��������������̣��۲쵽�������ǣ��䷴Ӧ�Ļ�ѧ����ʽΪ��
| �������� | ����� | ������Ư | Ư�� |
| �� �� | ��Ч����۹�������ζ | Ưϴ���ʹɫ�ʸ����� | ����Ư���������� |
| ��Ч�ɷ� | HCl | H2O2 | NaClO |
�١�����顱����ܡ����ܡ���������������ã�
�ڡ�����顱�롰Ư�������ܻ��ã�����������ײ���һ���ж����壮��Ư��������Ч�ɷ�NaClO����Ԫ�صĻ��ϼ��ǣ�
�ۡ�����顱�����ڴ���ʯ���������ǣ��û�ѧ����ʽ��ʾ����
����ʢ��������������Ư��Һ����Թ��У��������������̣��۲쵽�������ǣ��䷴Ӧ�Ļ�ѧ����ʽΪ��
��2010?��������ģ����һ�������ļ���Һ������������������Ӧ��������Һ��������������ҵ������Ĺ�ϵ����������ͼ���߱�ʾ���ǣ� ��

| ��� | �� | �� |
| 1 | HCl��Һ | NaOH��Һ |
| 2 | HCl��Һ | ����ʯ |
| 3 | H2SO4��Һ | п�� |
| 4 | Ca��OH��2��Һ | Na2CO3��Һ |
| 5 | CuSO4��Һ | ���� |
