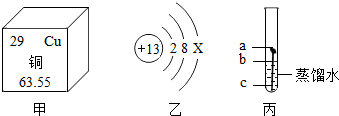��Ŀ����
����ͭ���������������еij���������
��1���������ֽ����У�________�ǵؿ��к������Ľ�����
��2��������������������������������õ�________�ԣ�
��3���ó�������Ҫ�ɷ�ΪFe2O3����һ����̼�����Ļ�ѧ����ʽΪ________��
��4����ҵ�ϳ�����������Ҫ�ɷ���������������������ѧ����ʽΪ2Al2O3 4Al+3O2������51t��������80%�������������Ͽ�������������________t��
4Al+3O2������51t��������80%�������������Ͽ�������������________t��
��5��ͭ���ڳ�ʪ�Ŀ�����Ҳ�������⣬ͭ�⣨�׳�ͭ�̣�����Ҫ�ɷ���Cu2��OH��2CO3���������ɷ�����ͭ������ͭ��ˮ��________��________��ͬ���õĽ����ͭ����ͭ��Ļ�ѧ����ʽΪ________��
�⣺��1���ؿ�����ǰ��λ��Ԫ���������������ʵؿ��к���������Ԫ���������ʣ�1���𰸣���
��2�����������õĵ����Ժ���չ�Թ������ڼӹ������������ʣ�2���𰸣����Ⱥ���չ
��3��һ����̼������������������Ӧ�������Ͷ�����̼���ʴ𰸣�3CO+Fe2O3 2Fe+3CO2
2Fe+3CO2
��4�����ݻ�ѧ��Ӧǰ��Ԫ�������غ������������е���Ԫ�ؼ���Ӧ�������е���Ԫ�أ�Ȼ������������Ļ�ѧʽ��Ԫ�ص�����Ϊ51t��80%�� =21.6t
=21.6t
��5������ͭ������ͭ��ˮ�������Ͷ�����̼��ͬ���õĽ���ʴ𰸣�2Cu+O2+H2O+CO2=Cu2��OH��2CO3
��������1�����õؿ���Ԫ�صĺ������
��2�����������е����Ժ���չ�Խ��
��3�����ݻ�ѧ����ʽ����д������д
��4������Ԫ�������غ�Ȼ����ݻ�ѧʽ����ijһԪ�صĺ���
��5������������Ϣ��ϻ�ѧ����ʽ����д������
������������ݻ�ѧʽ�ļ��㣻�����������������ƶϷ�Ӧ���Լ���ѧ����ʽ����д��
��2�����������õĵ����Ժ���չ�Թ������ڼӹ������������ʣ�2���𰸣����Ⱥ���չ
��3��һ����̼������������������Ӧ�������Ͷ�����̼���ʴ𰸣�3CO+Fe2O3
 2Fe+3CO2
2Fe+3CO2��4�����ݻ�ѧ��Ӧǰ��Ԫ�������غ������������е���Ԫ�ؼ���Ӧ�������е���Ԫ�أ�Ȼ������������Ļ�ѧʽ��Ԫ�ص�����Ϊ51t��80%��
 =21.6t
=21.6t��5������ͭ������ͭ��ˮ�������Ͷ�����̼��ͬ���õĽ���ʴ𰸣�2Cu+O2+H2O+CO2=Cu2��OH��2CO3
��������1�����õؿ���Ԫ�صĺ������
��2�����������е����Ժ���չ�Խ��
��3�����ݻ�ѧ����ʽ����д������д
��4������Ԫ�������غ�Ȼ����ݻ�ѧʽ����ijһԪ�صĺ���
��5������������Ϣ��ϻ�ѧ����ʽ����д������
������������ݻ�ѧʽ�ļ��㣻�����������������ƶϷ�Ӧ���Լ���ѧ����ʽ����д��

��ϰ��ϵ�д�
�����Ŀ