��Ŀ����
 ˫��ˮ������������Ư�ȷ��棬�ڶ������̵������£���Ѹ�ٷֽ�ų�������Ԭ��ʦ�ͻ�ѧС���ͬѧ�ǰ���ͼװ��ʵ�飮��ش�
˫��ˮ������������Ư�ȷ��棬�ڶ������̵������£���Ѹ�ٷֽ�ų�������Ԭ��ʦ�ͻ�ѧС���ͬѧ�ǰ���ͼװ��ʵ�飮��ش�
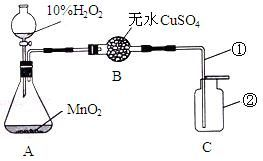
��1��װ���б�������������ǣ���________����________��
��2����ƿ�ڷ�����Ӧ�Ļ�ѧ����ʽ��________��
����ø�������Ʊ���������ѧ����ʽ��________��
��3����ʵ����ʹ����100g10%��H2O2��Һ����H2O2��Һ�к�����H2O2________g��
��4����ɫ����ˮ����ͭ��ˮ�������������Ԥ�Ȿʵ������У���ˮ����ͭ�Ƿ�������
________����ǡ�����
��5�����������ſ������ռ�������ԭ����________����ͼ�������ٲ��������ڵ�λ���Ƿ�
��ȷ��________����ǡ�����
��6��֤��C���������ռ����ķ�����________��
�⣺��1����ǿ�Գ��û�ѧ�������˽��ʶ����������ܡ�����ƿ
��2������������ȡ�����Ļ�ѧ����ʽ��ע��д��Ҫ��飬һ��Ҫ��ƽ���������䷽����
��3�������й������������������⣺100��10%=10�ˣ�
��4��˫��ˮѸ�ٷֽ�ų�������Ϊ���ȷ�Ӧ������ˮ�������ɣ�������ˮ����ͭ�������
��5����Ϊ�������ܶȴ��ڿ����ģ����������ſ������ռ����������ܲ���λ����ȷ��
��6�����������������������������ǵ�ľ�����ڼ���ƿ�ڸ�ȼ��
�ʴ�Ϊ����1���ٵ��� �ڼ���ƿ ��2��2H2O2�T2H2O+O2�� 2KMnO4�TK2MnO4+MnO2+O2�� ��3��10��4���� ��5�������ܶȱȿ����� �ǣ���ȷ�� ��6���������ǵ�ľ�����ڼ���ƿ�ڸ�ȼ
��������1��ͬѧ�ǶԳ��û�ѧ�������˽��ʶ�������ֻ�кܺõ���ʶ���ǣ���ʵ����ܵ���Ӧ�֣�
��2������������ȡ�����Ļ�ѧ����ʽ��ע��д��Ҫ��飬һ��Ҫ��ƽ��
��3�������й������������������⣺100��10%=10�ˣ����Ը�H2O2��Һ�к�����H2O2Ϊ10�ˣ�
��4��˫��ˮѸ�ٷֽ�ų�������Ϊ���ȷ�Ӧ������ˮ�������ɣ�������ˮ����ͭ�������
��5����Ϊ�������ܶȴ��ڿ����ģ����������ſ������ռ�������
��6�����������������������������ǵ�ľ�����ڼ���ƿ�ڸ�ȼ��
�������������ڿ�����������ȡ˼·��������й������������������⣮
��2������������ȡ�����Ļ�ѧ����ʽ��ע��д��Ҫ��飬һ��Ҫ��ƽ���������䷽����
��3�������й������������������⣺100��10%=10�ˣ�
��4��˫��ˮѸ�ٷֽ�ų�������Ϊ���ȷ�Ӧ������ˮ�������ɣ�������ˮ����ͭ�������
��5����Ϊ�������ܶȴ��ڿ����ģ����������ſ������ռ����������ܲ���λ����ȷ��
��6�����������������������������ǵ�ľ�����ڼ���ƿ�ڸ�ȼ��
�ʴ�Ϊ����1���ٵ��� �ڼ���ƿ ��2��2H2O2�T2H2O+O2�� 2KMnO4�TK2MnO4+MnO2+O2�� ��3��10��4���� ��5�������ܶȱȿ����� �ǣ���ȷ�� ��6���������ǵ�ľ�����ڼ���ƿ�ڸ�ȼ
��������1��ͬѧ�ǶԳ��û�ѧ�������˽��ʶ�������ֻ�кܺõ���ʶ���ǣ���ʵ����ܵ���Ӧ�֣�
��2������������ȡ�����Ļ�ѧ����ʽ��ע��д��Ҫ��飬һ��Ҫ��ƽ��
��3�������й������������������⣺100��10%=10�ˣ����Ը�H2O2��Һ�к�����H2O2Ϊ10�ˣ�
��4��˫��ˮѸ�ٷֽ�ų�������Ϊ���ȷ�Ӧ������ˮ�������ɣ�������ˮ����ͭ�������
��5����Ϊ�������ܶȴ��ڿ����ģ����������ſ������ռ�������
��6�����������������������������ǵ�ľ�����ڼ���ƿ�ڸ�ȼ��
�������������ڿ�����������ȡ˼·��������й������������������⣮

��ϰ��ϵ�д�
�����Ŀ
 ˫��ˮ������������Ư�ȷ��棬�ڶ������̵������£���Ѹ�ٷֽ�ų�������Ԭ��ʦ�ͻ�ѧС���ͬѧ�ǰ���ͼװ��ʵ�飮
˫��ˮ������������Ư�ȷ��棬�ڶ������̵������£���Ѹ�ٷֽ�ų�������Ԭ��ʦ�ͻ�ѧС���ͬѧ�ǰ���ͼװ��ʵ�飮 ˫��ˮ������������Ư�ȣ����ǹ������⣨H2O2����ˮ��Һ����ʢ������MnO2�������У����뺬H2O230%��˫��ˮ���ڳ����¼���Ѹ�ٷֽ�ų�������
˫��ˮ������������Ư�ȣ����ǹ������⣨H2O2����ˮ��Һ����ʢ������MnO2�������У����뺬H2O230%��˫��ˮ���ڳ����¼���Ѹ�ٷֽ�ų������� ˫��ˮ������������Ư�ȷ��棬�ڶ������̵������£���Ѹ�ٷֽ�ų�������Ԭ��ʦ�ͻ�ѧС���ͬѧ�ǰ�ͼװ��ʵ�飮��ش�
˫��ˮ������������Ư�ȷ��棬�ڶ������̵������£���Ѹ�ٷֽ�ų�������Ԭ��ʦ�ͻ�ѧС���ͬѧ�ǰ�ͼװ��ʵ�飮��ش�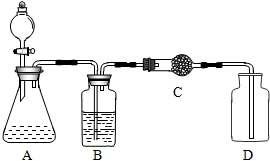 ˫��ˮ������������Ư�ȣ����ǹ������⣨H2O2����ˮ��Һ������������30%����Һ�Զ�������Ϊ������Ѹ�ٷֽ��������2H2O2
˫��ˮ������������Ư�ȣ����ǹ������⣨H2O2����ˮ��Һ������������30%����Һ�Զ�������Ϊ������Ѹ�ٷֽ��������2H2O2 ˫��ˮ������������Ư�ȷ��棬���ǹ������⣨H2O2������Һ����ʵ���������H2O2��Һ��ȡ������MnO2���������������ķ�Ӧ�ǣ�2H2O2
˫��ˮ������������Ư�ȷ��棬���ǹ������⣨H2O2������Һ����ʵ���������H2O2��Һ��ȡ������MnO2���������������ķ�Ӧ�ǣ�2H2O2