��Ŀ����
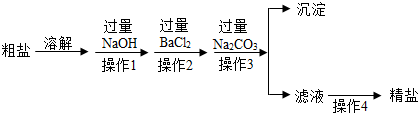
ͨ����ˮɹ�ƿɵô��Σ����γ�NaCl�⣬������MgCl2��CaCl2��MgSO4�Լ���ɳ�����ʣ��������Ʊ����ε�ʵ�鷽����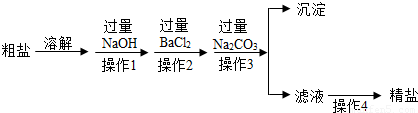
�ش��������⣺
��1������1�У�����NaOH��Һ����ʱ����Ӧ�Ļ�ѧ����ʽΪ �� ��
��2������2�У����������ᱵ��Һ����BaCl2��Һ��ԭ���� ��
��3������Na2CO3��ȥ������������ ��
��4�����в���3���ж�Na2CO3�����ķ����� ��
��5����Һ�У�������������Լ��������Լ��������� ��
��6���ڲ���4�У���Ҫ��������������ʱ���õ�������������̨������Ȧ�����ƾ��ơ��������� ��
��7�������εIJ���ƫ�ͣ����ܵ�ԭ���� ��
A������ʱ��ֽ������
B������ʱ�й��彦��
C���ܽ����ʱ�������ˮ�����㣬����û����ȫ�ܽ⣮
���𰸡�������þ�����ܹ����������ӽ������������þ������
���ᡢ�����ζ�������ˮ����������Ӳ����׳�ȥ��
�����ӡ��������ܺ�̼������ӽ������̼��ơ�̼�ᱵ������
̼��������ܺ������ӽ������ˮ�Ͷ�����̼��
�������ơ�̼���ƶ��ܺ�ϡ���ᷴӦ��
��������ʱ�����ܻ�Ӱ��ʵ������
����⣺��1�����������ܺ��Ȼ�þ������þ��Ӧ�ֱ�����������þ���Ȼ��ơ�������þ�������ƣ���Ӧ�Ļ�ѧ����ʽ�ֱ�Ϊ��2NaOH+MgCl2�TMg��OH��2��+2NaCl��
MgSO4+2NaOH�TMg��OH��2��+Na2SO4��
���2NaOH+MgCl2�TMg��OH��2��+2NaCl��MgSO4+2NaOH�TMg��OH��2��+Na2SO4��
��2�����������ᱵ��Һ����BaCl2��Һ������Ǽ������ᱵ��Һ������һϵ�з�Ӧ�����õ����Ȼ����лẬ�������ƣ�
������������������ƣ�
��3������Na2CO3���Գ�ȥ�����������Ǹ����Ӻͱ����ӣ�
���Ca2+��Ba2+��
��4���ж�Na2CO3�����ķ����ǣ�ȡ������Һ���Թ��У��μ�ϡ���ᣬ�����ݲ�����
���ȡ������Һ���Թ��У��μ�ϡ���ᣬ�����ݲ�����
��5����Һ�У��������������ϡ���ᣬ�������е��������ơ�̼���Ʒ�Ӧ�ֱ������Ȼ��ƺ�ˮ���Ȼ��ơ�ˮ�Ͷ�����̼���Ӷ��õ��Ȼ�����Һ��
���ϡ���ᣮ
��6������ʱ���õ�������������̨������Ȧ�����ƾ��ơ���������������
���������
��7��A������ʱ��ֽ��������ʹ���εIJ���ƫ�ߣ�
B������ʱ�й��彦������ʹ���εIJ���ƫ�ͣ�
C���ܽ����ʱ�������ˮ�����㣬����û����ȫ�ܽ⣬��ʹ���εIJ���ƫ�ͣ�
���B��C��
������������Ҫ�����˳��ӵķ������̣��ǽ��ѵ�һ����Ŀ�����ʱҪ��������������ʵ����ʣ������ʵ����ʳ��������з������жϣ��Ӷ�������ȷ�Ľ��
���ᡢ�����ζ�������ˮ����������Ӳ����׳�ȥ��
�����ӡ��������ܺ�̼������ӽ������̼��ơ�̼�ᱵ������
̼��������ܺ������ӽ������ˮ�Ͷ�����̼��
�������ơ�̼���ƶ��ܺ�ϡ���ᷴӦ��
��������ʱ�����ܻ�Ӱ��ʵ������
����⣺��1�����������ܺ��Ȼ�þ������þ��Ӧ�ֱ�����������þ���Ȼ��ơ�������þ�������ƣ���Ӧ�Ļ�ѧ����ʽ�ֱ�Ϊ��2NaOH+MgCl2�TMg��OH��2��+2NaCl��
MgSO4+2NaOH�TMg��OH��2��+Na2SO4��
���2NaOH+MgCl2�TMg��OH��2��+2NaCl��MgSO4+2NaOH�TMg��OH��2��+Na2SO4��
��2�����������ᱵ��Һ����BaCl2��Һ������Ǽ������ᱵ��Һ������һϵ�з�Ӧ�����õ����Ȼ����лẬ�������ƣ�
������������������ƣ�
��3������Na2CO3���Գ�ȥ�����������Ǹ����Ӻͱ����ӣ�
���Ca2+��Ba2+��
��4���ж�Na2CO3�����ķ����ǣ�ȡ������Һ���Թ��У��μ�ϡ���ᣬ�����ݲ�����
���ȡ������Һ���Թ��У��μ�ϡ���ᣬ�����ݲ�����
��5����Һ�У��������������ϡ���ᣬ�������е��������ơ�̼���Ʒ�Ӧ�ֱ������Ȼ��ƺ�ˮ���Ȼ��ơ�ˮ�Ͷ�����̼���Ӷ��õ��Ȼ�����Һ��
���ϡ���ᣮ
��6������ʱ���õ�������������̨������Ȧ�����ƾ��ơ���������������
���������
��7��A������ʱ��ֽ��������ʹ���εIJ���ƫ�ߣ�
B������ʱ�й��彦������ʹ���εIJ���ƫ�ͣ�
C���ܽ����ʱ�������ˮ�����㣬����û����ȫ�ܽ⣬��ʹ���εIJ���ƫ�ͣ�
���B��C��
������������Ҫ�����˳��ӵķ������̣��ǽ��ѵ�һ����Ŀ�����ʱҪ��������������ʵ����ʣ������ʵ����ʳ��������з������жϣ��Ӷ�������ȷ�Ľ��

��ϰ��ϵ�д�
�����Ŀ