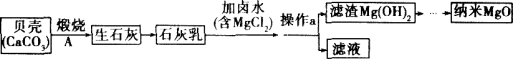��Ŀ����
����Ŀ�������죬Сǿ������Ҫ�����������Сǿȥ�̵����һ�����Сǿ��ϸ���˰�װ˵������ͼ�������������ʣ�
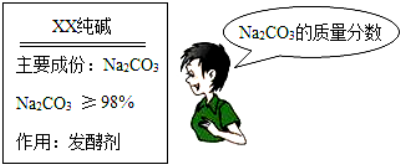
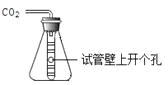
�ص�ѧУ����ȡ���Ӽ��������һС��������Ʒ����ʵ�飺ȷ��ȡ5.5g��Ʒ�����ձ��У��ٵμ��������պ���ȫ��Ӧ������CO2����ˮ��������ȥϡ����25g���õ���Һ����Ϊ28.3g����������ˮ�Ҳ������ᷴӦ������
��1������CO2��������
��2��ͨ�������жϴ�����Ʒ��̼���Ƶ����������Ƿ����װ˵�����������������ȷ��0.1����
���𰸡���1��2.2g ��2�����װ˵������
��������������������Ҫע�⣺��ȷ��Ŀ���⣬�ҵ��������ͻ�ƿ��������������̼���������ǽ��������ͻ�ƿ�����������Ʒ�м���ϡ���ᣬ������Һ��������С����Ʒ������������ͣ����ٵ��������Ƕ�����̼��������������û�ѧ����ʽ���ɶ�����̼����������̼���Ƶ�������Ȼ�����̼���Ƶ�����������������ǩ��̼���Ƶ����������Ƚϼ��ɣ�
��1������CO2���������Ϊ5.5g��25g��28.3g��2.2g��
��2���⣺��5.5g��Ʒ�к���̼���Ƶ�����Ϊx��
Na2CO3��2HCl��2NaCl��CO2����H2O
106 44
x 2.2g
106�U44��x�U2.2g
���x��5.3g
����Ʒ��̼���Ƶ���������Ϊ![]()
��Ϊ96.4����98�����������װ˵��������
���ԣ�

 ��ʱѵ���������������ϵ�д�
��ʱѵ���������������ϵ�д� �ƸԾ���Ȥζ����ϵ�д�
�ƸԾ���Ȥζ����ϵ�д� ����С����ҵ��ϵ�д�
����С����ҵ��ϵ�д�