��Ŀ����
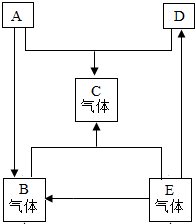
A��B��C��D��E�dz��л�ѧ�г����IJ�ͬ�������ʣ����ʰ����ʡ�������ᡢ��η��ࣩ����֪A�ǵ��ʣ�C�Ǻ���ɫ���壻E��ˮ��Һ��ʹ��̪��Һ��Ϊ��ɫ���Ρ�ͼ�С�—����ʾ��������������֮����Է�����Ӧ����������ʾ��ijһ���ʿ��Ƶ���һ���ʣ����ַ�Ӧ������P��Ӧ��������ȥ�����ش��������⣺
��1��д���������ʵĻ�ѧʽ��A ��E ��
��2����Eת��ΪB�Ļ�ѧ����ʽΪ ����Cת��ΪA��ԭ���ڹ�ҵ�ϳ����� ��

�� Fe Na2CO3����K2CO3�� �� Na2CO3+Ca(OH)2 = CaCO3��+2NaOH(��K2CO3+Ca(OH)2 = CaCO3��+2KOH�����������𰸼���) ����

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 ��֪A��B��C��D��E�dz��л�ѧ�������������ʣ�����A��D�Ǻ�ɫ���壬AΪ��ɫ���嵥�ʣ�DΪ��ɫ��������������к�������ʹ����㷺�Ľ���Ԫ�أ�B��C��E����ɫ���壬��������һ�������µ�ת����ϵ����ͼ��ʾ��������Ӧ�������������ȥ����
��֪A��B��C��D��E�dz��л�ѧ�������������ʣ�����A��D�Ǻ�ɫ���壬AΪ��ɫ���嵥�ʣ�DΪ��ɫ��������������к�������ʹ����㷺�Ľ���Ԫ�أ�B��C��E����ɫ���壬��������һ�������µ�ת����ϵ����ͼ��ʾ��������Ӧ�������������ȥ���� 23����֪A��B��C��D��E�dz��л�ѧ�ﳣ�����������ʣ�������һ���������ܷ�����ͼ��ʾ��ת�������з�Ӧ���Ǹ��ֽⷴӦ��E���������ЧӦ����Ҫ���壮
23����֪A��B��C��D��E�dz��л�ѧ�ﳣ�����������ʣ�������һ���������ܷ�����ͼ��ʾ��ת�������з�Ӧ���Ǹ��ֽⷴӦ��E���������ЧӦ����Ҫ���壮 A��B��C��D��E�dz������ʣ�����B��E�ǿ������������壬C��������Ҫ�ɷ֣���ͼ��ʾ��ֱ�߱�ʾ����ܹ���Ӧ����ͷ��ʾת���ķ���
A��B��C��D��E�dz������ʣ�����B��E�ǿ������������壬C��������Ҫ�ɷ֣���ͼ��ʾ��ֱ�߱�ʾ����ܹ���Ӧ����ͷ��ʾת���ķ��� ��2013?���ݶ�ģ��A��B��C��D��E�dz��л�ѧ�г����IJ�ͬ�������ʣ����ʰ����ʡ�������ᡢ��η��ࣩ����֪A�ǵ��ʣ�C�Ǻ���ɫ���壻E��ˮ��Һ��ʹ��̪��Һ��Ϊ��ɫ���Σ�ͼ�С�-����ʾ��������������֮����Է�����Ӧ����������ʾ��ijһ���ʿ��Ƶ���һ���ʣ����ַ�Ӧ������P��Ӧ��������ȥ�����ش��������⣺
��2013?���ݶ�ģ��A��B��C��D��E�dz��л�ѧ�г����IJ�ͬ�������ʣ����ʰ����ʡ�������ᡢ��η��ࣩ����֪A�ǵ��ʣ�C�Ǻ���ɫ���壻E��ˮ��Һ��ʹ��̪��Һ��Ϊ��ɫ���Σ�ͼ�С�-����ʾ��������������֮����Է�����Ӧ����������ʾ��ijһ���ʿ��Ƶ���һ���ʣ����ַ�Ӧ������P��Ӧ��������ȥ�����ش��������⣺ A��B��C��D��E�dz��л�ѧ�г�����5�����ʣ����Ƕ�����һ����ͬ��Ԫ�أ���ͼ��ʾ������֮���ת����ϵ�����У�AΪʳ�ε���Ҫ�ɷ֣�B�к���Ԫ�أ�D��E����Һ������ɫ��
A��B��C��D��E�dz��л�ѧ�г�����5�����ʣ����Ƕ�����һ����ͬ��Ԫ�أ���ͼ��ʾ������֮���ת����ϵ�����У�AΪʳ�ε���Ҫ�ɷ֣�B�к���Ԫ�أ�D��E����Һ������ɫ��