��Ŀ����
 ��2007?���£�ijͬѧΪ���о�Ӱ��������ᷴӦ���ʵ����أ�������һϵ�е�̽��ʵ�飮ʵ������ͼ��ʾ��ͼ��ÿ��б�߱�ʾ���ý����Ͷ�Ӧ��ķ�Ӧʱ�����������������Ĺ�ϵ����ͼ�е���Ϣ���ǿ��Է�����Ӱ��������ᷴӦ���ʵ������У�
��2007?���£�ijͬѧΪ���о�Ӱ��������ᷴӦ���ʵ����أ�������һϵ�е�̽��ʵ�飮ʵ������ͼ��ʾ��ͼ��ÿ��б�߱�ʾ���ý����Ͷ�Ӧ��ķ�Ӧʱ�����������������Ĺ�ϵ����ͼ�е���Ϣ���ǿ��Է�����Ӱ��������ᷴӦ���ʵ������У���
�����Ļ�ԶԽ�������ķ�Ӧ������Ӱ��
�����Ļ�ԶԽ�������ķ�Ӧ������Ӱ��
����
��������ĽӴ�����Խ�������ķ�Ӧ������Ӱ��
��������ĽӴ�����Խ�������ķ�Ӧ������Ӱ��
����
����Һ�����ʵ�����������С�Խ�������ķ�Ӧ������Ӱ��
����Һ�����ʵ�����������С�Խ�������ķ�Ӧ������Ӱ��
������������Ӱ�컯ѧ��Ӧ���ʵ������������棺һ�Ƿ�Ӧ������ʣ������Խ�������ᷴӦԽ�죮����������أ���Ҫ���¶ȡ�Ũ�ȡ�ѹǿ���������Ӵ�����ȷ������
����⣺��ͼ��֪�������������Ľ�����Բ�ͬӰ��������ᷴӦ���ʣ����ۺ���Ƭ����ĽӴ������ͬ������Ҳ��Խ�������ķ�Ӧ������Ӱ�죻ϡ�����Ũ�Ȳ�ͬҲ��Խ�������ķ�Ӧ������Ӱ�죮
�ʴ�Ϊ���ٽ����Ļ�ԶԽ�������ķ�Ӧ������Ӱ�죻
�ڽ�������ĽӴ�����Խ�������ķ�Ӧ������Ӱ�죻
������Һ�����ʵ�����������С�Խ�������ķ�Ӧ������Ӱ�죮
�ʴ�Ϊ���ٽ����Ļ�ԶԽ�������ķ�Ӧ������Ӱ�죻
�ڽ�������ĽӴ�����Խ�������ķ�Ӧ������Ӱ�죻
������Һ�����ʵ�����������С�Խ�������ķ�Ӧ������Ӱ�죮
���������⿼��Ӱ�컯ѧ��Ӧ���ʵ������������أ�ͬʱҲ������ѧ������ͼ��Ӧ��ͼ���������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 ��2007?���£�ijУ��ѧ��ȤС���ѧУ������ˮ��״����չ��һϵ�е�ʵ�����о���������ͬѧ�ǵIJ���ʵ�����ݣ���������ǵ��о����ش��������⣺
��2007?���£�ijУ��ѧ��ȤС���ѧУ������ˮ��״����չ��һϵ�е�ʵ�����о���������ͬѧ�ǵIJ���ʵ�����ݣ���������ǵ��о����ش��������⣺ ��2007?���£����йز���ͳ�ƣ�ÿ��ȫ������������ʧ�ĸ���Լռ������������ķ�֮һ��Ϊ�ˣ�ijͬѧ��������ʴ�������������о���Ȥ�����������ͼ��ʾ��ʵ�飮�ݴ���ش��������⣺
��2007?���£����йز���ͳ�ƣ�ÿ��ȫ������������ʧ�ĸ���Լռ������������ķ�֮һ��Ϊ�ˣ�ijͬѧ��������ʴ�������������о���Ȥ�����������ͼ��ʾ��ʵ�飮�ݴ���ش��������⣺
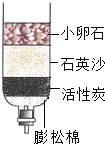
 ��2007?���£������к�������ȫ�����ĵ�ˮ������ֳ�أ�Ϊ̽������Ļ�ѧ�ɷ֣�С������Google���������ڻ������ϲ�ѯ��֪��
��2007?���£������к�������ȫ�����ĵ�ˮ������ֳ�أ�Ϊ̽������Ļ�ѧ�ɷ֣�С������Google���������ڻ������ϲ�ѯ��֪��