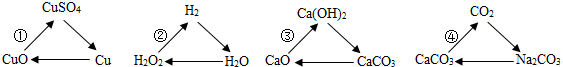
��Ŀ����
��2013?���Ƹۣ�ij�о���С���ͬѧ�õ��ˮ�ķ����ⶨˮ����ɺ�������⣺���ⶨˮ����ɻ��������ķ����𣿡��������ۣ��õ��˿϶��Ĵ𰸣�������һ��Դ�չ��̽����
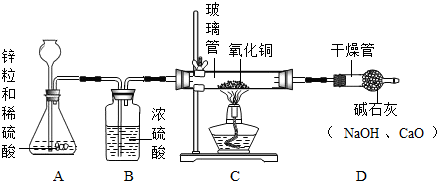
����Ʒ�������ͬѧ����������ԭ����ͭ��ԭ������ͼװ�ü�ҩƷ����ʵ�飨�����淶����װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ
��ʵ��̽�������������й����ɺ�ɫת��Ϊ��ɫʱ��ʵ��ã��ٵIJ����ܺ����й�����������ڷ�Ӧ�������1.6g����װ��D�ĸ���ܵ��������ڷ�Ӧ��������1.82g���ô��������ˮ��H��OԪ�ص�������Ϊ
����������������������������ֵ��ƫ���ͬѧ��Ϊ����װ�ô���ȱ�ݣ��˹۵�õ��˴�ҵ���ͬ����Դ˸Ľ��ķ�����
�����ⷢ�֡���ͬѧ��С�Ľ���Ӧ���������ɫ����a���䵽����ϡ�������ˣ����ֳ����к�ɫ���� b���⣬��Һ����ɫ����ɫ��Ϊ��ɫ��
��������⡿ͭ��ϡ�����Dz���Ӧ�ģ�������Һ����ɫΪʲô������أ�
���������ϡ���CuO����ԭ�Ĺ����л���Cu2O���ɣ�Cu2OҲ�ܱ���ԭ��Cu��
�ڹ����Ǻ�ɫ�ģ�����ϡ����ķ�ӦΪCu2O+H2SO4=CuSO4+Cu+H2O
���ó����ۡ���ɫ����a�к���Cu2O
��������������ɫ����a�к���Cu2O �Ƿ��Ӱ��ˮ�ⶨ���
��������롿��Ժ�ɫ����a�ijɷ֣�С��ͬѧ���������ǣ���Cu2O ��Cu�� �������
����չ���롿��ͬѧ�����øĽ���װ�ü�ҩƷ����ʵ�飬ͨ��������Ӧǰ����������ķ�����ȷ�����ֲ�����������ѳƵã��ٲ����ܵ��������ڷ�Ӧǰ���ɫ����a�Ͳ����ܵ�����������ȫ��Ӧ������Ϊ������Ҫ����
����ʦ�㲦����ȡһ�������ĺ�ɫ����a��������ϡ�����ַ�Ӧ�����ˡ�ϴ�ӡ�������ٳ�����ɫ����b��������Ҳ����ȷ�����ֲ��������
������������С��ͬѧ�ٴ�ʵ�飬���ǿ�ʼ��ȡ����Ϊ3.6g�ĺ�ɫ����a����������ϡ������ȫ��Ӧ�õ���ɫ����bΪ2g������3.6g��ɫ����a����Cu2O��������������ͭ������������Cu2O+H2SO4=CuSO4+Cu+H2O���м��㣬д��������̣���Ȼ��ͨ����ʵ�ʵĹ���2g���бȽϣ��жϲ���

����Ʒ�������ͬѧ����������ԭ����ͭ��ԭ������ͼװ�ü�ҩƷ����ʵ�飨�����淶����װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ
Zn+H2SO4�TZnSO4+H2��
Zn+H2SO4�TZnSO4+H2��
��ϴ��ƿB��Ũ���������Ϊ����ˮ
����ˮ
��ʵ��̽�������������й����ɺ�ɫת��Ϊ��ɫʱ��ʵ��ã��ٵIJ����ܺ����й�����������ڷ�Ӧ�������1.6g����װ��D�ĸ���ܵ��������ڷ�Ӧ��������1.82g���ô��������ˮ��H��OԪ�ص�������Ϊ
11��80
11��80
����ˮ�Ļ�ѧʽ���H��OԪ�ص�������Ϊ1��8
1��8
�� ����������������������������ֵ��ƫ���ͬѧ��Ϊ����װ�ô���ȱ�ݣ��˹۵�õ��˴�ҵ���ͬ����Դ˸Ľ��ķ�����
��Dװ�ú���������һ�������
��Dװ�ú���������һ�������
���������ɵ�ˮ��ȫ��D�м�ʯ�����գ�װ���ڿ�����ˮ������CO2���Բ��ƣ���С��ͬѧ�øĽ���װ������ʵ��õ�����ȷ����� �����ⷢ�֡���ͬѧ��С�Ľ���Ӧ���������ɫ����a���䵽����ϡ�������ˣ����ֳ����к�ɫ���� b���⣬��Һ����ɫ����ɫ��Ϊ��ɫ��
��������⡿ͭ��ϡ�����Dz���Ӧ�ģ�������Һ����ɫΪʲô������أ�
���������ϡ���CuO����ԭ�Ĺ����л���Cu2O���ɣ�Cu2OҲ�ܱ���ԭ��Cu��
�ڹ����Ǻ�ɫ�ģ�����ϡ����ķ�ӦΪCu2O+H2SO4=CuSO4+Cu+H2O
���ó����ۡ���ɫ����a�к���Cu2O
��������������ɫ����a�к���Cu2O �Ƿ��Ӱ��ˮ�ⶨ���
��Ӱ��
��Ӱ��
���Ӱ�족��Ӱ�족���� ��������롿��Ժ�ɫ����a�ijɷ֣�С��ͬѧ���������ǣ���Cu2O ��Cu�� �������
Cu2O
Cu2O
����չ���롿��ͬѧ�����øĽ���װ�ü�ҩƷ����ʵ�飬ͨ��������Ӧǰ����������ķ�����ȷ�����ֲ�����������ѳƵã��ٲ����ܵ��������ڷ�Ӧǰ���ɫ����a�Ͳ����ܵ�����������ȫ��Ӧ������Ϊ������Ҫ����
Dװ��
Dװ��
�������� ����ʦ�㲦����ȡһ�������ĺ�ɫ����a��������ϡ�����ַ�Ӧ�����ˡ�ϴ�ӡ�������ٳ�����ɫ����b��������Ҳ����ȷ�����ֲ��������
������������С��ͬѧ�ٴ�ʵ�飬���ǿ�ʼ��ȡ����Ϊ3.6g�ĺ�ɫ����a����������ϡ������ȫ��Ӧ�õ���ɫ����bΪ2g������3.6g��ɫ����a����Cu2O��������������ͭ������������Cu2O+H2SO4=CuSO4+Cu+H2O���м��㣬д��������̣���Ȼ��ͨ����ʵ�ʵĹ���2g���бȽϣ��жϲ���
��
��
��������ٻ�ڣ���������п�ܺ�ϡ���ᷢ���û���Ӧ��Ũ���������ˮ�ԣ�
�����к���ˮ�Ͷ�����̼��
�������й�����ٵ������������ɵ�ˮ����Ԫ�ص���������ɫ����a�к���Cu2O��Ӱ���ˮ�IJⶨ�����
�����ܵ���������Ӧǰ���ɫ����a�Ͳ����ܵ����������Ѿ������������֪������ˮ���������Ϳ���ȷ����ɫ����a�ijɷ֣�
���ݻ�ѧ����ʽ�������ȷ����ɫ����a�ijɷ֣�
�����к���ˮ�Ͷ�����̼��
�������й�����ٵ������������ɵ�ˮ����Ԫ�ص���������ɫ����a�к���Cu2O��Ӱ���ˮ�IJⶨ�����
�����ܵ���������Ӧǰ���ɫ����a�Ͳ����ܵ����������Ѿ������������֪������ˮ���������Ϳ���ȷ����ɫ����a�ijɷ֣�
���ݻ�ѧ����ʽ�������ȷ����ɫ����a�ijɷ֣�
����⣺����Ʒ�����
п��ϡ���ᷴӦ����������п����������Ӧ�Ļ�ѧ����ʽΪ��Zn+H2SO4�TZnSO4+H2����
���Zn+H2SO4�TZnSO4+H2����
ϴ��ƿB��Ũ���������������ˮ��
�������ˮ��
��ʵ��̽����
�ô��������ˮ����Ԫ�ص�������1.6g����Ԫ�ص�������1.82g-1.6g=0.22g����ˮ��H��OԪ�ص�������Ϊ��0.22g��1.6g=11��80����ˮ�Ļ�ѧʽ���H��OԪ�ص�������Ϊ��
��1��2������16��1��=1��8��
���11��80��1��8��
������������
��Ϊ�����е�ˮ��������̼�����˸���ܣ�Ӱ���˽��������Ӧ����Dװ�ú���������һ������ܣ������ܹ���ֹ�����е�ˮ�Ͷ�����̼����D�У�
�����Dװ�ú���������һ������ܣ�
������������
��ɫ����a�к���Cu2O��Ӱ��ˮ�ⶨ�������Ϊ�������й�����ٵ������������ɵ�ˮ����Ԫ�ص�����������ijɷ��أ�
�����Ӱ�죮
��������롿�����Ӧ����������ͭ��
���Cu2O��
����չ���롿
��Ϊ��ȫ��Ӧ��ͭ������ͨ���������Լ�����������֪����Ӧ���ɵ�ˮ�����������ݻ�ѧ����ʽ���������Ӧ����ͭ���������ٺ�ͨ�������������ͭ�������Ƚϣ��Ϳ���ȷ���������ɣ�
���Dװ�ã�
������������
��3.6g��ɫ����a����Cu2O������ȫ��Ӧ������ͭ������ΪX��
Cu2O+H2SO4=CuSO4+Cu+H2O
144 64
3.6g X
=
X=1.6g��
��õ��ĺ�ɫ����2g������˵����ɫ�����к���������ͭ��ͭ��
����٣�
п��ϡ���ᷴӦ����������п����������Ӧ�Ļ�ѧ����ʽΪ��Zn+H2SO4�TZnSO4+H2����
���Zn+H2SO4�TZnSO4+H2����
ϴ��ƿB��Ũ���������������ˮ��
�������ˮ��
��ʵ��̽����
�ô��������ˮ����Ԫ�ص�������1.6g����Ԫ�ص�������1.82g-1.6g=0.22g����ˮ��H��OԪ�ص�������Ϊ��0.22g��1.6g=11��80����ˮ�Ļ�ѧʽ���H��OԪ�ص�������Ϊ��
��1��2������16��1��=1��8��
���11��80��1��8��
������������
��Ϊ�����е�ˮ��������̼�����˸���ܣ�Ӱ���˽��������Ӧ����Dװ�ú���������һ������ܣ������ܹ���ֹ�����е�ˮ�Ͷ�����̼����D�У�
�����Dװ�ú���������һ������ܣ�
������������
��ɫ����a�к���Cu2O��Ӱ��ˮ�ⶨ�������Ϊ�������й�����ٵ������������ɵ�ˮ����Ԫ�ص�����������ijɷ��أ�
�����Ӱ�죮
��������롿�����Ӧ����������ͭ��
���Cu2O��
����չ���롿
��Ϊ��ȫ��Ӧ��ͭ������ͨ���������Լ�����������֪����Ӧ���ɵ�ˮ�����������ݻ�ѧ����ʽ���������Ӧ����ͭ���������ٺ�ͨ�������������ͭ�������Ƚϣ��Ϳ���ȷ���������ɣ�
���Dװ�ã�
������������
��3.6g��ɫ����a����Cu2O������ȫ��Ӧ������ͭ������ΪX��
Cu2O+H2SO4=CuSO4+Cu+H2O
144 64
3.6g X
| 144 |
| 64 |
| 3.6g |
| X |
X=1.6g��
��õ��ĺ�ɫ����2g������˵����ɫ�����к���������ͭ��ͭ��
����٣�
������ͨ���������ȷ�����ʵ���ɣ����ǽ���������������֮һ�������ص㡢�����ѵ㣬Ҫ��ϸ��ᡢȫ�����գ�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ