��Ŀ����
��ѧ������ϢϢ��أ���1������ʳƷ����������ҪӪ������

��2�����м����У���ȱ�ٸ�Ԫ���йص���
A���������� B��٪��֢ C�����Ͳ� D��ƶѪ
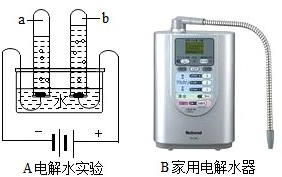
��3����ͼAʵ��ͨ��ʱ��a�Թ���Һ��ʼ��ԡ�b�Թ���Һ������ԣ����ô�ԭ����Ƶ�ͼB��ʾ�ļ��õ��ˮ�����ɵ�����Բ�ͬ��ˮ������;���±�C���ش��������⣮
| pH | ˮ��Ӧ�� |
| 10.0 | �����߲� |
| 9.5 | �ճ����� |
| 9.0 | ��� |
| 6.0 | ϴͷ�� |
| 5.5 | ϴ������ |
д��ͼA��������Ӧ�Ļ�ѧ����ʽ
��4�������ǵĺ�ˮ�ü���ˮ�����о�����������õ���ˮ����
��������1���߲ˡ�ˮ����Ҫ�ṩ��������Ҫ��ά���أ�
��2��ȱ��Ԫ�����������ɡ����Ͳ���
��3�����ˮ�õ���������������ҺpH����Һ����ԵĹ�ϵΪpH��7����Һ�����ԣ�pH=7����Һ�����ԣ�pH��7����Һ�ʼ��ԣ�
��4������ˮ����Ҫͨ�����˳�ȥˮ�в����Թ������ʣ��������Ե��������ܽ���ˮ�У��õ��IJ��Ǵ�����ˮ��
��2��ȱ��Ԫ�����������ɡ����Ͳ���
��3�����ˮ�õ���������������ҺpH����Һ����ԵĹ�ϵΪpH��7����Һ�����ԣ�pH=7����Һ�����ԣ�pH��7����Һ�ʼ��ԣ�
��4������ˮ����Ҫͨ�����˳�ȥˮ�в����Թ������ʣ��������Ե��������ܽ���ˮ�У��õ��IJ��Ǵ�����ˮ��
����⣺��1��ͼ����ʾ����ҪΪˮ�����߲ˣ����Ǹ������������ά���أ�
�ʴ�Ϊ��ά���أ�
��2���ƶԹ�������������������Ҫ���ã�Ӥ��ȱ�ƣ������Ͳ�����ͯȱ�ƣ�Ӱ������ķ����ͳ����ӣ�����ȱ�ƣ����γɹ������ɣ��������ۣ�
�ʴ�Ϊ��AC��
��3��ͼA��ʾΪ���ˮ��ʵ��װ�ã�ˮ��ͨ�������¿ɷֽ�������������������C�����ڡ��ճ����á���ˮ�Ķ�ӦpH����Ϊ9.5��7��˵����ˮ�ʼ��ԣ�
�ʴ�Ϊ��2H2O
2H2��+O2�������ԣ�
��4������ˮ��ֻ�ǰѻ��ǵĺ�ˮ�еĹ��岻�������ʹ��˶���ȥ������Щ���ܵ����ʵ���Ȼ������ˮ�У�������õ���ˮ�����ж������ʣ�Ϊ����
�ʴ�Ϊ������
�ʴ�Ϊ��ά���أ�
��2���ƶԹ�������������������Ҫ���ã�Ӥ��ȱ�ƣ������Ͳ�����ͯȱ�ƣ�Ӱ������ķ����ͳ����ӣ�����ȱ�ƣ����γɹ������ɣ��������ۣ�
�ʴ�Ϊ��AC��
��3��ͼA��ʾΪ���ˮ��ʵ��װ�ã�ˮ��ͨ�������¿ɷֽ�������������������C�����ڡ��ճ����á���ˮ�Ķ�ӦpH����Ϊ9.5��7��˵����ˮ�ʼ��ԣ�
�ʴ�Ϊ��2H2O
| ||
��4������ˮ��ֻ�ǰѻ��ǵĺ�ˮ�еĹ��岻�������ʹ��˶���ȥ������Щ���ܵ����ʵ���Ȼ������ˮ�У�������õ���ˮ�����ж������ʣ�Ϊ����
�ʴ�Ϊ������
������������Ҫ�����һЩ��������صĻ���֪ʶ�����������⣮

��ϰ��ϵ�д�
�����Ŀ

 ��ѧ������ϢϢ��أ�
��ѧ������ϢϢ��أ�